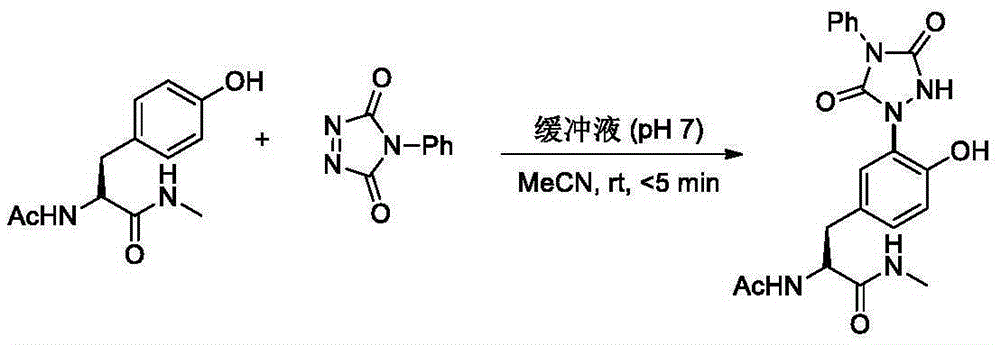
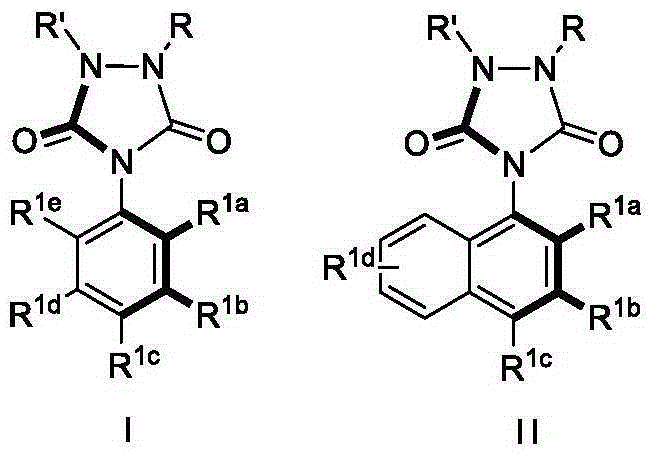
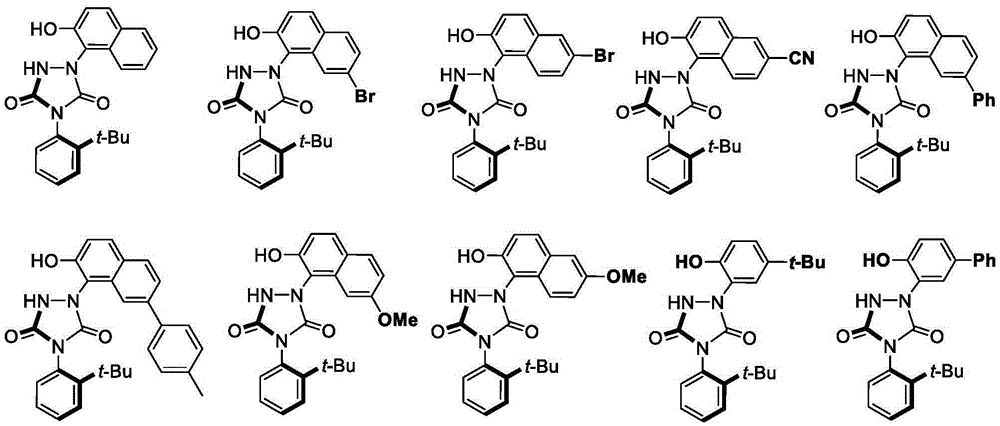
Urazole chiral axis compound and catalytic asymmetric synthesis method thereof
A synthetic method and axial chiral technology, which is applied in the field of ureazoline axial chiral compounds and their catalytic asymmetric synthesis, achieving the effects of easy preparation, good stability, and good enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058]
[0059] Dissolve 0.12 mmol of compound 2a and chiral catalyst C7 (5 mol%, 0.005 mmol) in 2 mL of ether in a Schlenk tube, stir the resulting solution at -78°C for 10 min, then add 0.10 mmol of compound 1a, and react for 30 min until the red color disappears After monitoring the reaction with TLC, the reaction solution was acidified with 6N hydrochloric acid, concentrated, and subjected to silica gel column chromatography (CH 2 Cl 2 ~CH 2 Cl 2 / Acetone=10 / 1) The product 3a was obtained after purification.
[0060] At room temperature, 0.2 mmol of compound 3a and 2.0 mmol of methyl iodide were dissolved in 1 mL of 1,4-dioxane, 0.4 mmol of potassium carbonate was added, and the resulting solution was stirred at room temperature for 5 h, and subjected to silica gel column chromatography (EA / PE=1: 2) The product 6a is obtained.
[0061] Compound 3a: 82% yield; 99% ee;
[0062] HPLC conditions: Daicel CHIRALPAKAD-H column, hexane (n-hexane) / i-PrOH=85 / 15, 0.8mL / min, T...
Embodiment 2
[0067] According to the method of Example 1, the solvent is ethylene dichloride, the catalyst consumption is 10 mmol%, the catalyst is C1, and the reaction is completed within 5 minutes. Compound 3a: 57% yield; 25% ee.
Embodiment 3
[0069] According to the method of Example 1, the solvent is ethylene dichloride, the catalyst consumption is 10 mmol%, the catalyst is C2, and the reaction is completed within 5 minutes. Compound 3a: 59% yield; 5% ee.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com