Targeted drug composition for co-loading amphotericin B and adriamycin and application of targeted drug composition
A technology of amphotericin and composition, which is applied in the field of targeted drug composition co-carrying amphotericin B and doxorubicin, which can solve the problem of increased drug consumption, toxic and side effects, and economic expenditure on patients' physical and psychological burden, and treatment cycle Prolongation and other issues, to achieve good biocompatibility, avoid drug resistance, and improve treatment efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Preparation of mannose-modified β-cyclodextrin propionate
[0033] (1) Synthesis of seven (6-azido-6-deoxy-2,3-di-O-propionyl)-β-cyclodextrin: 1.5 g of seven (6-azido-6-de O)-β-cyclodextrin and 4.5g of 4-dimethylaminopyridine were dissolved in 36mL of pyridine, and 8.2mL of propionic anhydride was added, then the temperature of the system was raised to 60°C, and stirred for 48 hours under nitrogen protection; the reaction After the solution was cooled to normal temperature, 200 mL of water was added, extracted 3 times with 150 mL of ethyl acetate, then excess anhydrous sodium sulfate was added to remove water, after filtration, the solvent was distilled off under reduced pressure, and finally purified by silica gel column (petroleum ether: acetic acid ethyl ester = 4:1);
[0034] (2) Synthesis of mannose-modified β-cyclodextrin propionate (Man7-β-CD-C3, MCC): 208 mg of seven (6-azido-6-deoxy-2,3-di- (O-propionyl)-β-cyclodextrin and 200 mg of propargyl D-mann...
Embodiment 2
[0041] Embodiment 2 A kind of preparation of pharmaceutical composition
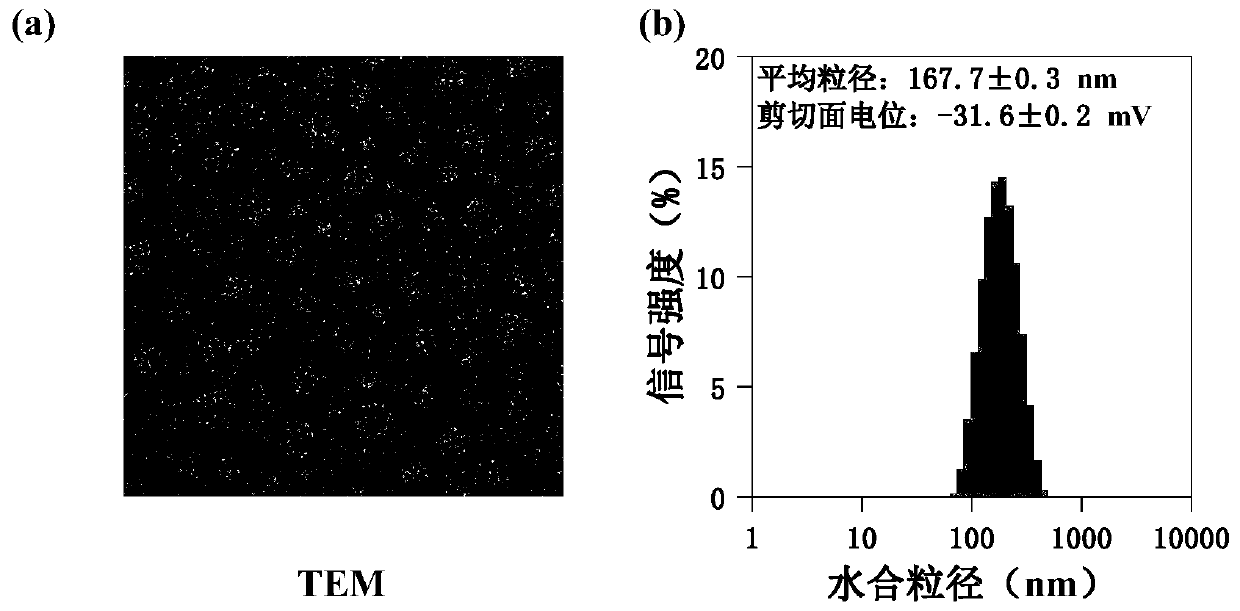
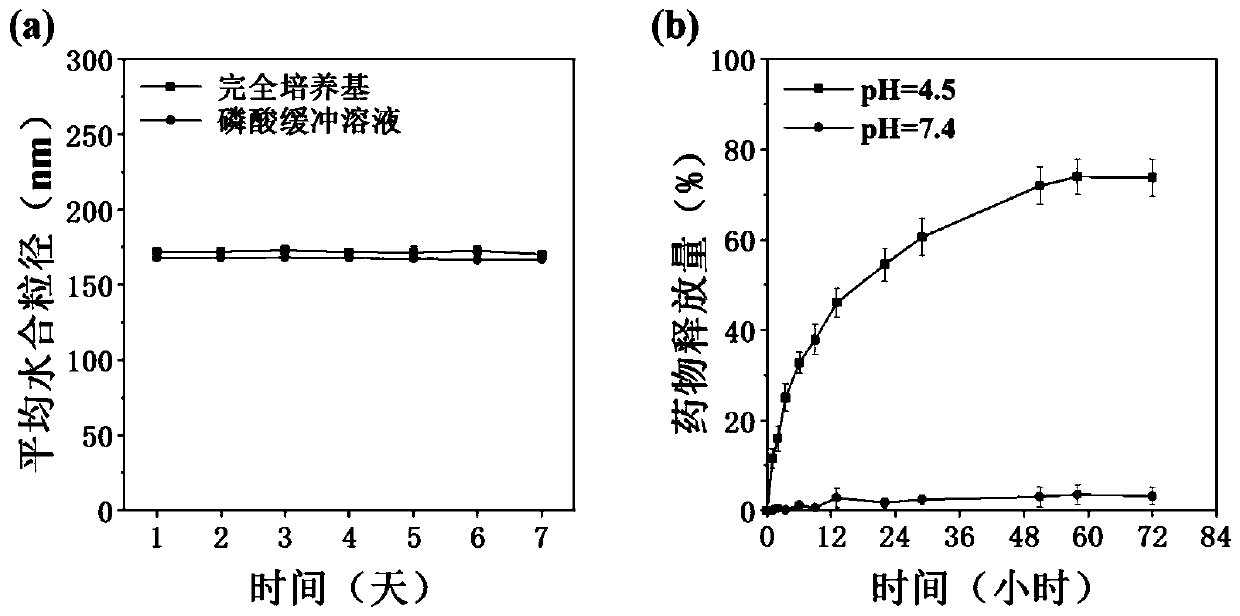
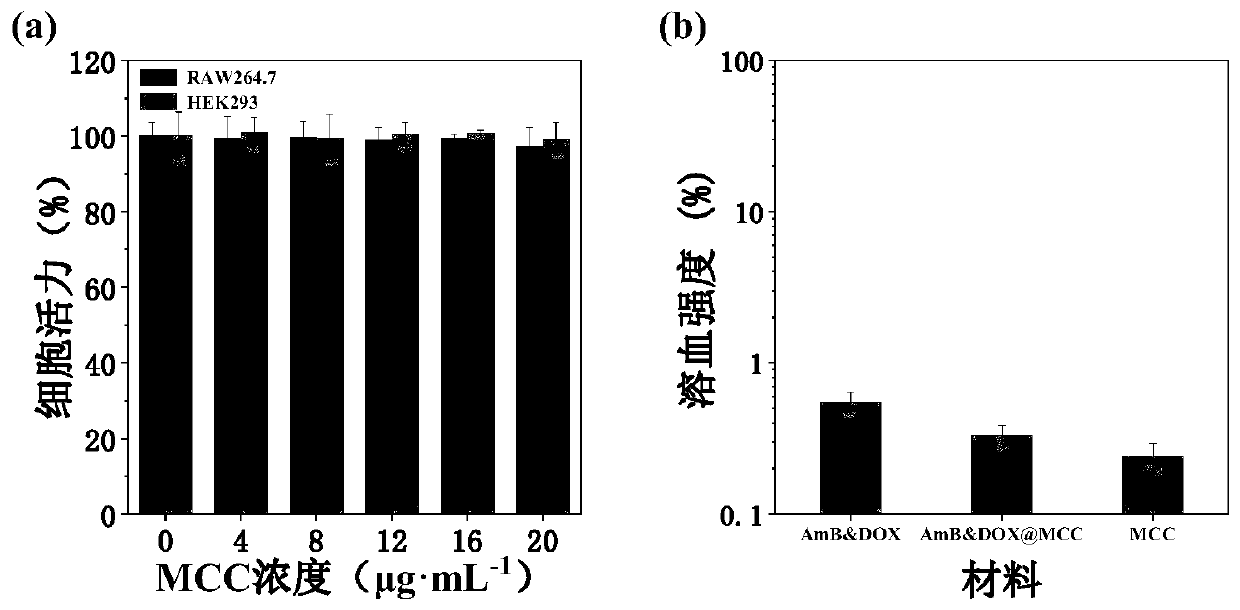
[0042] The mannose-modified β-cyclodextrin propionate, 2 mg of amphotericin B, and 2 mg of doxorubicin prepared in Example 1 of 10 mg were weighed and dissolved in dimethyl sulfoxide (MCC:AmB:DOX molar ratio 1:0.5:0.5), the mass concentration of the solution is 0.1mg / mL, after fully dissolving and mixing uniformly, drop into 1 times the volume of ultrapure water, mix evenly, move into a dialysis bag and dialyze with ultrapure water, filter , to obtain nano micelles; the volume ratio of the dialysis system is 1:100-1:500, and the number of times of changing the dialysate is 5-10 times; the particle size range of the nano micelles is 100-200nm .
Embodiment 3
[0043] Embodiment 3 A kind of preparation of pharmaceutical composition
[0044] The mannose-modified β-cyclodextrin propionate, 2 mg of amphotericin B, and 2 mg of doxorubicin prepared in Example 1 of 10 mg were weighed and dissolved in dimethyl sulfoxide (MCC:AmB:DOX molar ratio 1:0.5:0.5), the mass concentration of the solution is 1mg / mL, after fully dissolving and mixing uniformly, drop into 1 times the volume of ultrapure water, mix evenly, move it into a dialysis bag and dialyze with ultrapure water, filter it, That is, nano micelles are obtained; the volume ratio of the dialysis system is 1:100-1:500, and the number of dialysate changes is 5-10 times; the particle size range of the nano micelles is 100-200nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com