Hydrophobia-tetanus double titer human immunoglobulin, method for preparing same and application thereof in pharmacy
A technology of human immunoglobulin and immunoglobulin, which is applied in the direction of antiviral immunoglobulin, antibacterial immunoglobulin, immunoglobulin from serum, etc., can solve the problems of inconvenient clinical use, single potency, and high cost, and achieve Large social and economic benefits, simple and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
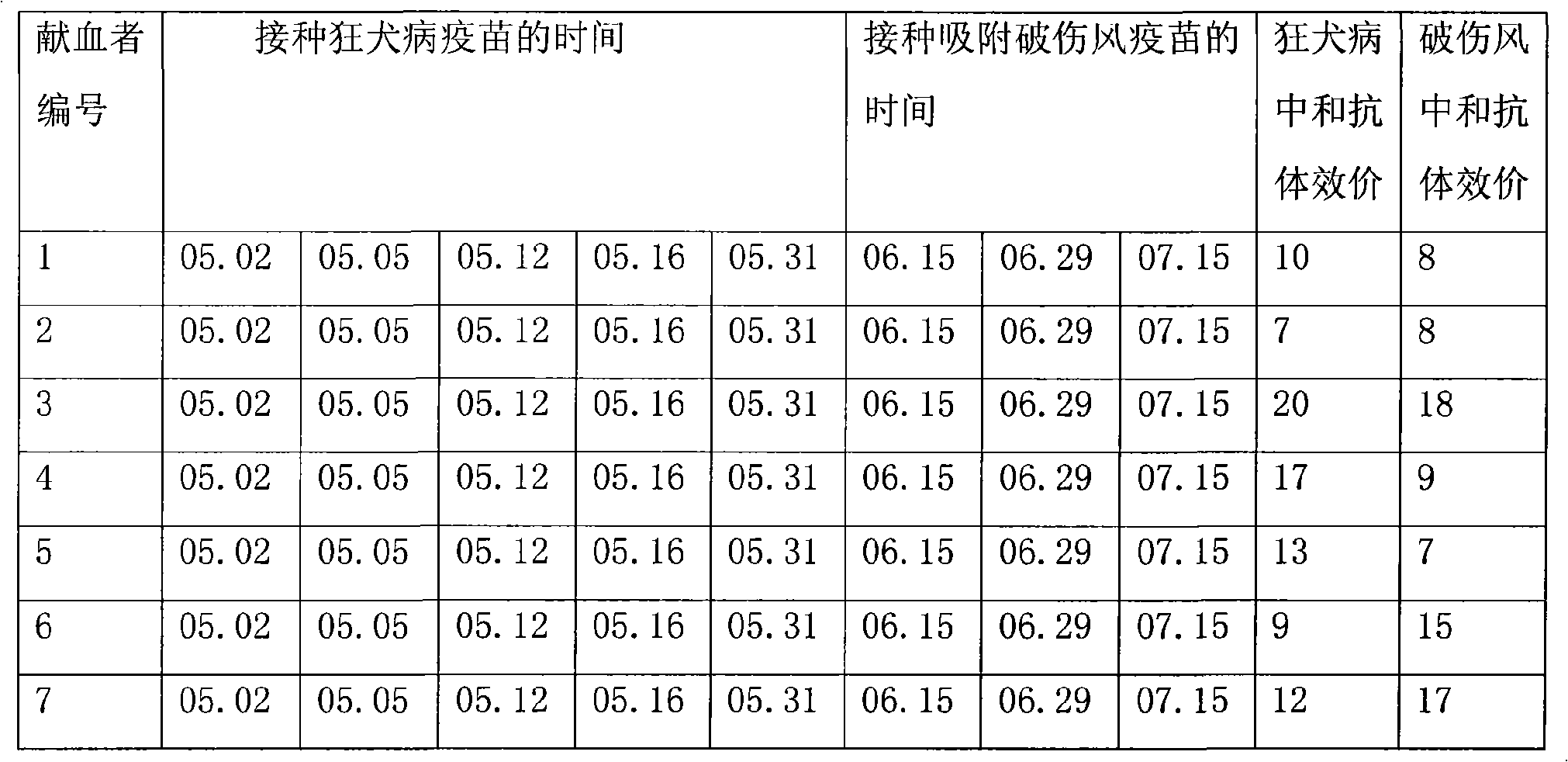
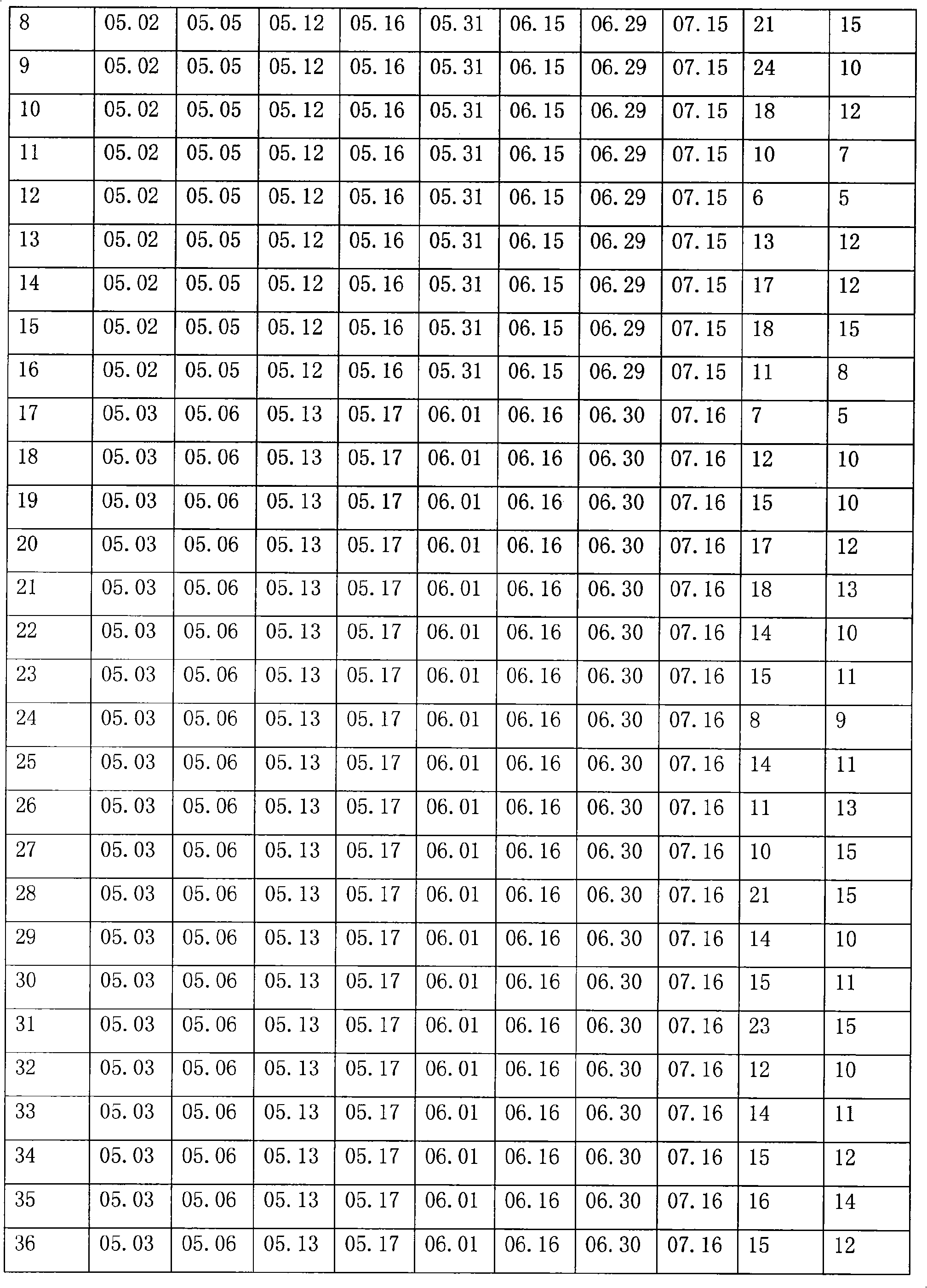
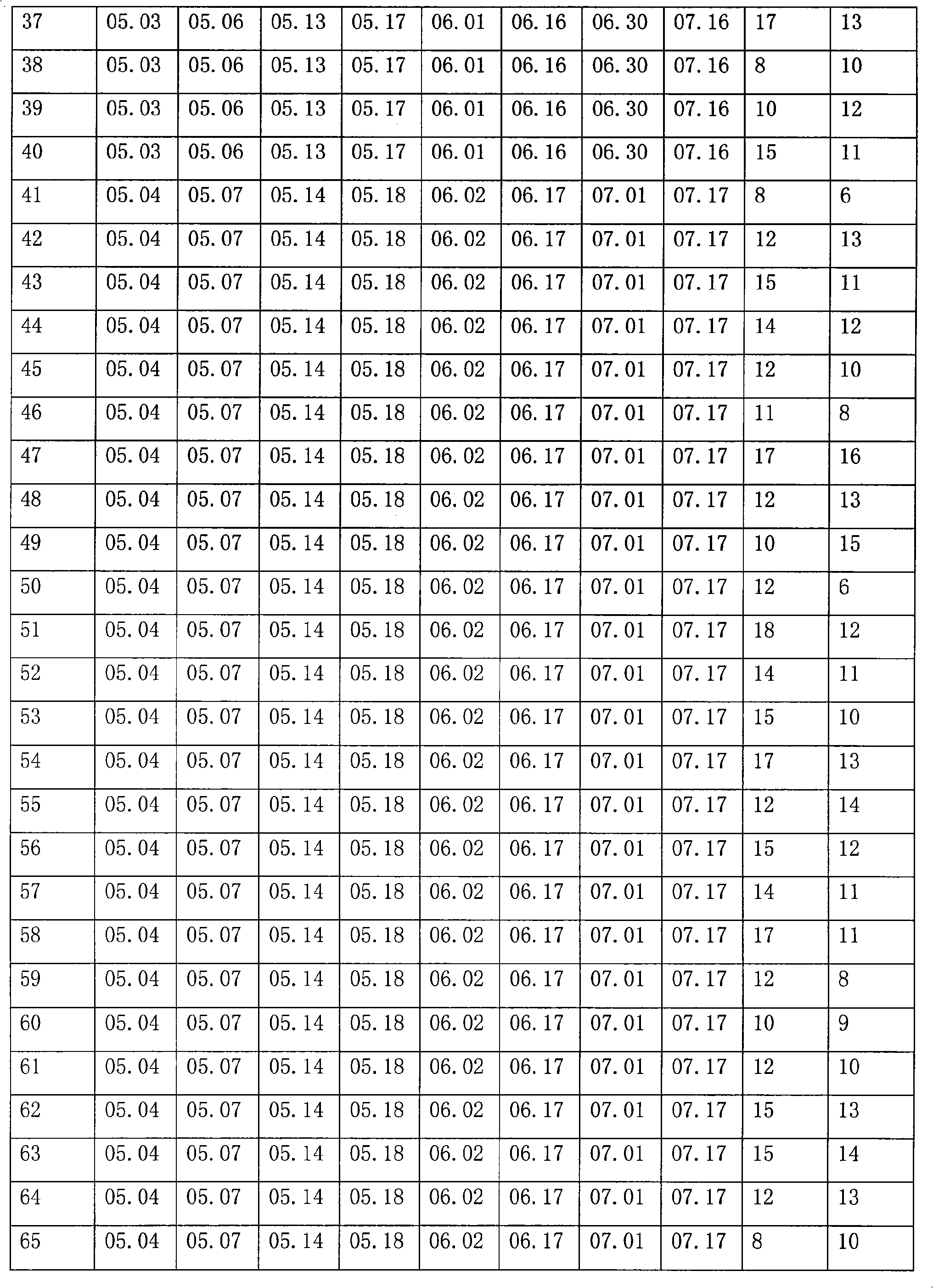
[0023] 1. Immunity of plasma donors:
[0024] 263 plasma donors who met the national blood donation standards were selected, and they were inoculated with rabies vaccine (produced by Liaoning Chengda Biology Co., Ltd., batch number: 20081001, dose: 0.5 ml / branch) 1ml, 0.5ml, 0.5ml, 0.5ml, 1ml. 15 days after the completion of rabies vaccination, apply the adsorbed tetanus vaccine (produced by Wuhan Institute of Biological Products, batch number 20081002, dose 0.5mL / bottle), according to the dosage of 0.5mL on days 0, 14±2, and 28±5 Immunization, and establish detailed immunization records. The national blood collection standard includes the following items: the protein content detected by the biuret method is greater than or equal to 55g / L, the alanine aminotransferase (Lai's method) is not higher than 25 units, the hepatitis B surface antigen is negative, and the syphilis is negative , HIV-1 / HIV-2 antibody negative, HCV antibody negative.
[0025]
[0026]
[0027] ...
Embodiment 2
[0081] Clinical application evaluation: (1) 120 patients in the experimental group were bitten or scratched by rabies or other mad animals, aged 2-61 years, with an average of 23.7 years; Immunoglobulin is used to prevent the occurrence of rabies and tetanus. The dosage is calculated according to the titer of rabies neutralizing antibody 20IU / kg body weight, and it is applied once. Results: No adverse reactions occurred; no rabies and tetanus occurred in continuous observation for 1 year. (2) In the control group, 47 patients who were bitten or scratched by rabies or other mad animals were exposed, aged 3-65 years, with an average of 21.4 years old; rabies patient immunoglobulin produced by Hualan Biotechnology (batch number: 200803001, specification: 200IU / 2.0ml / bottle) is used to prevent the occurrence of rabies, and the dosage is calculated according to the rabies neutralizing antibody titer 20IU / kg body weight, once applied; simultaneously use the tetanus human immunoglob...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Antibody titer | aaaaa | aaaaa |
| Potency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com