Methods of treating squamous cell carcinomas with farnesyltransferase inhibitors
A technology of squamous cell carcinoma, transferase, applied in the field of cancer treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0383] Enhanced in vivo efficacy of tipirfarnib in HNSCC with high H-Ras expression or high H / N+K ratio
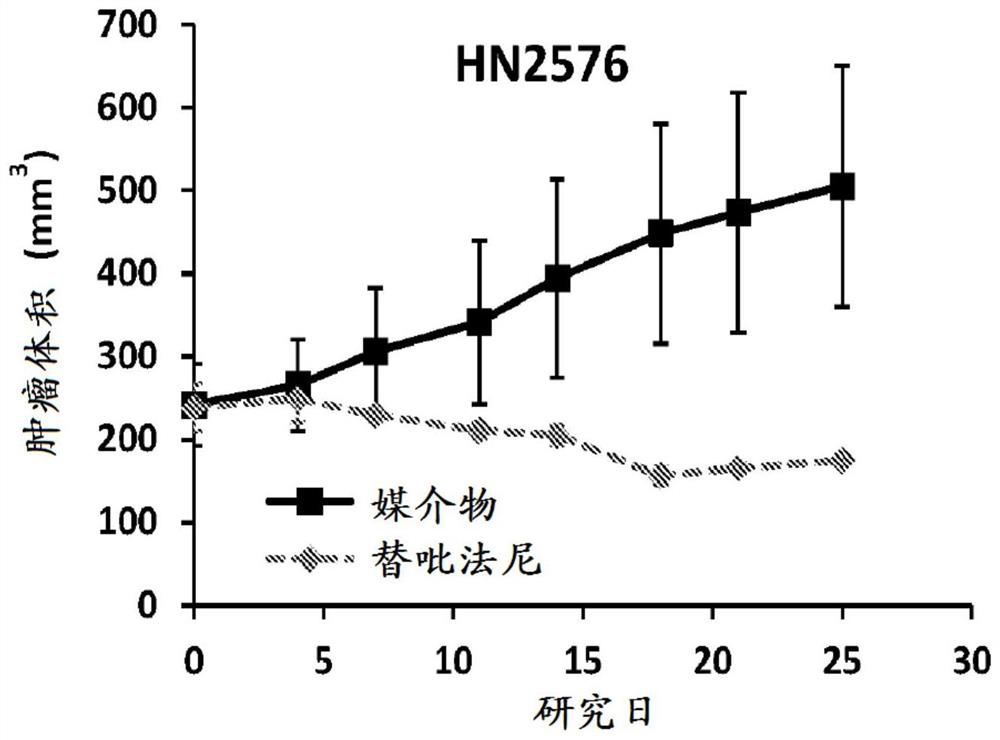
[0384] Tumors were generated by inoculating primary human tumor model fragments (2-3 mm in diameter) subcutaneously in the right flank of female BALB / c nude mice or Nu / nu mice (6-8 weeks). When the average tumor size reaches about 250-350mm 3 , the mice were randomly divided into treatment groups. Animals were dosed with tipifarnib vehicle (20% w / v hydroxypropyl-β-cyclodextrin) or tipifarnib at a dose of 80 mg / kg BID PO for 3-4 weeks and tumors were measured weekly Size twice.
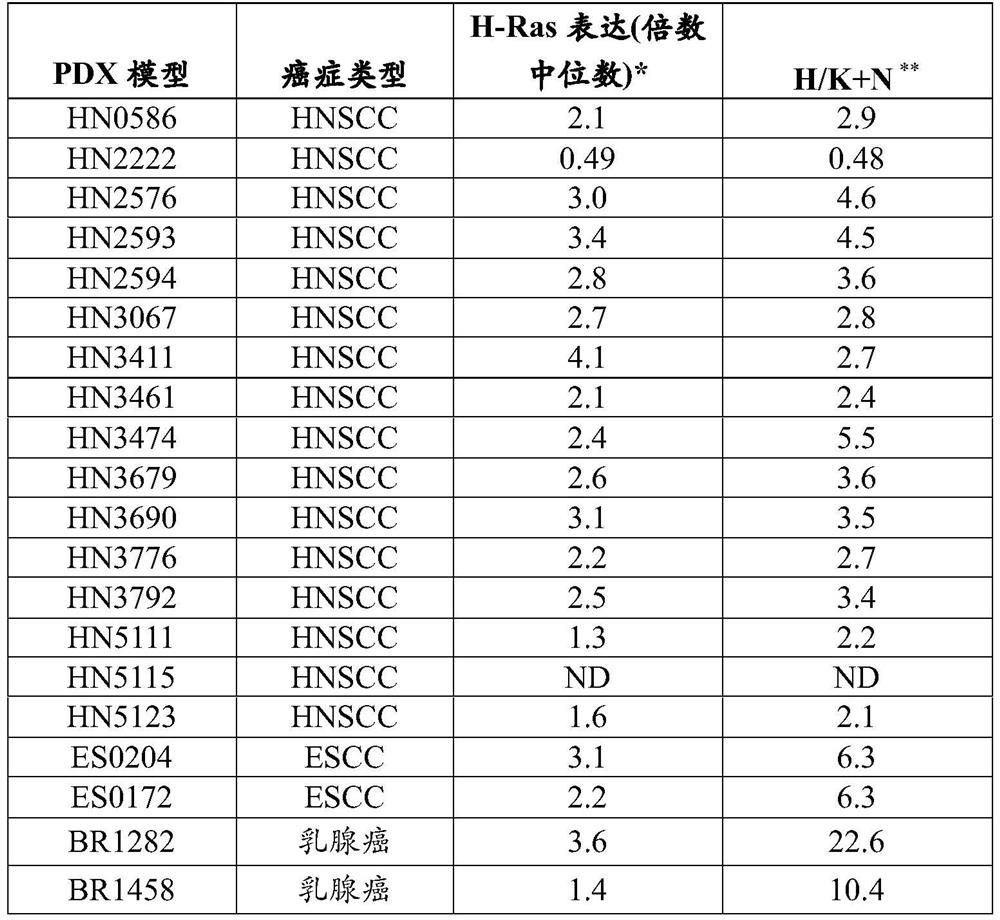
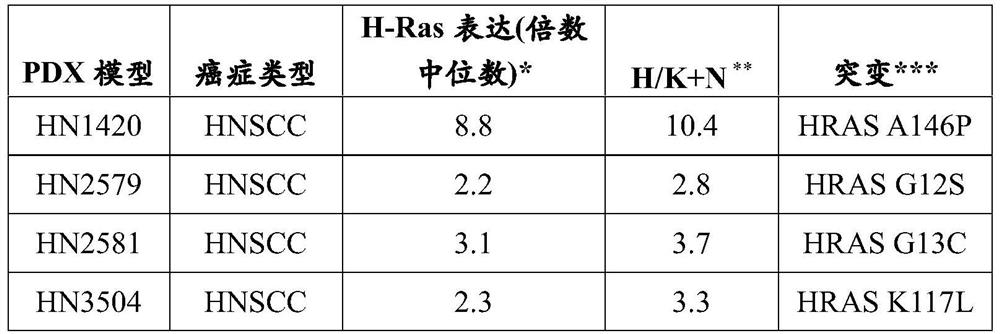
[0385] Selected patient-derived xenograft (PDX) models of HNSCC were used to determine the ability of tipirfarnib to inhibit tumor growth. Selected models express different expression levels of H-Ras, or have different ratios of higher H-Ras expression / combined expression of K-Ras and N-Ras ("H / K+N ratio"). The expression levels of H-Ras, K-Ras and N-Ras in these PDX models were determined by R...
Embodiment II
[0394] Enhanced in vivo efficacy of tipifarnib in esophageal squamous cell carcinoma but not breast cancer with high H / N+K ratio
[0395] As described in Example 1, a PDX model of esophageal squamous cell carcinoma (ESCC) (ES0204 or ES0172) or breast cancer (BR1282 or BR1458) was inoculated subcutaneously in the flank of nude mice. The H / K+N ratios for these models are detailed in Table 1. like Figure 2A and Figure 2B As shown in , tipirfarnib effectively inhibits with a relatively high H / K+N ratio ( Figure 2A Tumor growth in both ES0172 (p=0.02) and ES0204 (p=0.04) models of ). However, although BR1282 (p=0.53) or BR1458 (p=0.28) breast cancer PDX models also had relatively high H / K+N ratios ( Figure 2C and Figure 2D ), but this effect was not observed in these models. Therefore, high H-Ras expression or high H / K+N ratio is specifically associated with the efficacy of FTIs (such as tipifarnib) in SCC such as HNSCC, ESCC and urothelial carcinoma, but not other non-s...
Embodiment III
[0397] Synergistic effect of tipifarnib and second therapy in HNSCC with high H-Ras expression or high H / N+K ratio
[0398] PDX HNSCC models (HN3411 and HN2594) were inoculated subcutaneously in the flank of mice as described in Example 1. Following tumor development, the mice are administered vehicle, tipifarnib, the second active agent, or a combination of tipifarnib and the second active agent. The second agent is the alkylating agent cisplatin, the EGFR inhibitor cetuximab, or the CDK inhibitor palbociclib. As indicated, cisplatin ( Figure 3B ) and palbociclib ( Figure 3C ) do not have any activity when used alone. However, both cisplatin and palbociclib caused further inhibition of tumor growth when combined with tipifarnib compared to tipifarnib alone ( Figure 3B and Figure 3C ). Thus, tipifarnib not only directly inhibits tumor growth in HNSCC, but also sensitizes tumors to other treatments such as cisplatin or palbociclib.
[0399] Both cetuximab and tipifar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com