Application of farnesyltransferase inhibitors in preparation of medicine for facilitated cholinergic nerve system
A farnesyltransferase and nervous system technology, applied in the field of anti-dementia drugs, can solve the problems of high curative effect and less side effects, and achieve the effects of enhancing activity and expression, improving cognitive function decline, and improving dementia behavior
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
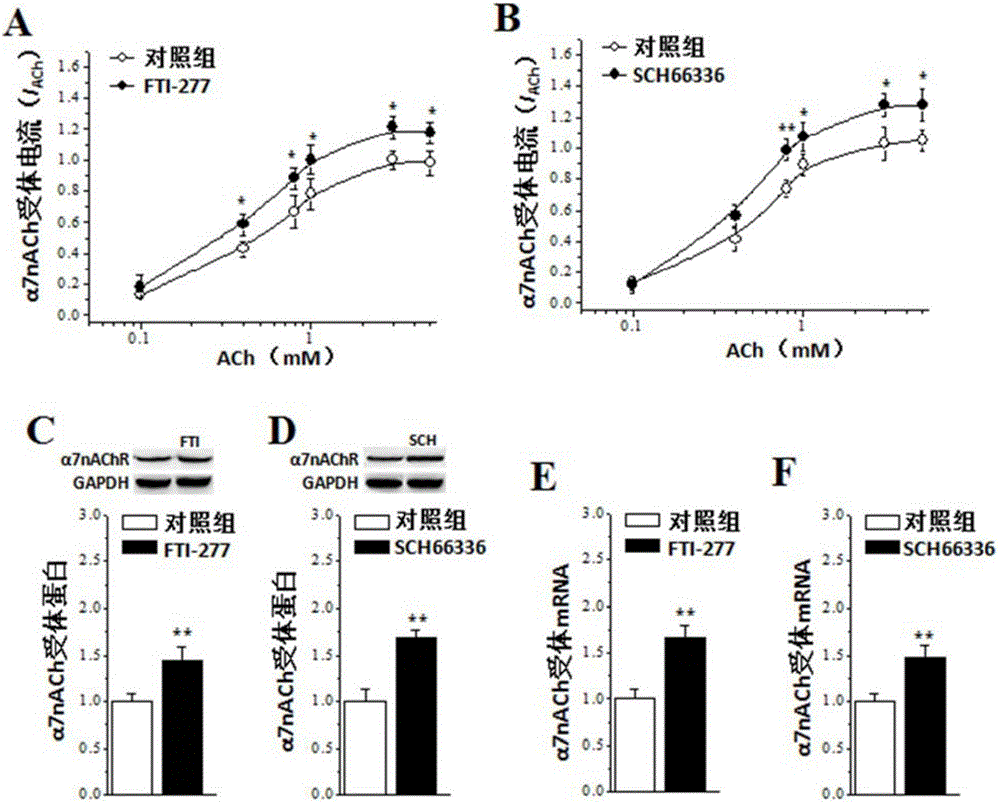
[0033] Example 1: Farnesyltransferase inhibitors can enhance the activity and expression of α7nACh receptors
[0034] Experimental main materials:
[0035] 30-35-day-old ICR mice were purchased from the Experimental Animal Center of Nanjing Medical University. The animals were kept in the Experimental Animal Center of Nanjing Medical University, and maintained in an environment with a temperature of 23±2°C, a humidity of 55±5%, and a 12:12h light / dark cycle. They have free access to food and water.
[0036] Drugs and Reagents
[0037] Farnesyltransferase inhibitors (farnesyltransferase inhibitors, FTI): ①Farnesyltransferase inhibitor (FTI, FTI-277) purchased from Calbiochem, USA (Cat#344555), produced emulsion in 5% Tween 80 or dissolved in dimethyl sulfoxide (DMSO), Perform intraperitoneal injection (50mg / kg) or brain slice incubation (1μM). ② Lonafarnib (SCH66336) was purchased from Medchemexpress (Cat#HY-15136) in the United States, dissolved in dimethyl sulfoxide (DMSO),...
Embodiment 2
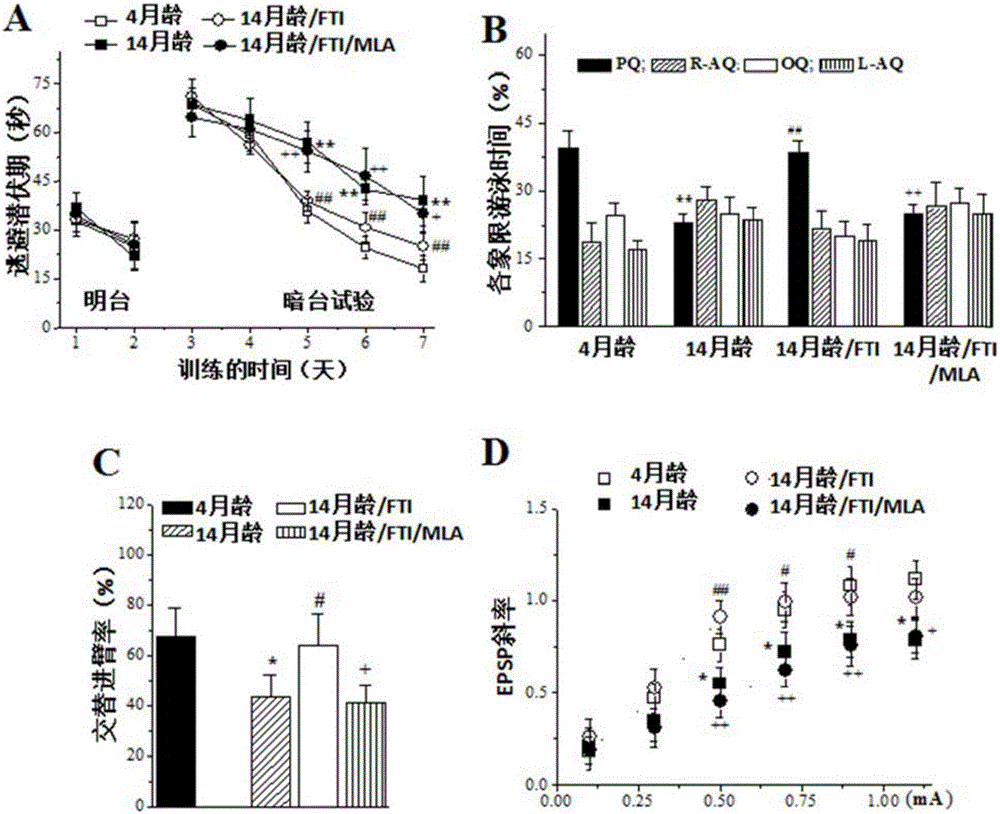
[0050] Example 2: Inhibitors of farnesyltransferase improve the cognitive function of aged mice—anti-senile dementia.
[0051] Experimental main materials
[0052]4-month-old and 14-month-old male ICR mice (weight 27-30g, SPF grade) were provided by Shanghai Slack Experimental Animal Co., Ltd., license number: SCXK (Shanghai) 2007-0005, certificate number: 2007000517888. Animals were raised in a clean-grade animal room at a temperature of 22-25°C, using SPF-grade special pellet feed for rats and mice and SPF-grade sterilized litter (provided by Nanjing Anlimo Technology Co., Ltd.). 12-hour (6:00-18:00) light-dark cycle, free access to food and water (Nanjing Medical University Animal Center). All operating instruments and materials that come into contact with animals are sterilized by ultraviolet light. Experimental animal operators have been specially trained and have a certificate of qualification for using experimental animals. The preparation of experimental liquids is ca...
Embodiment 3
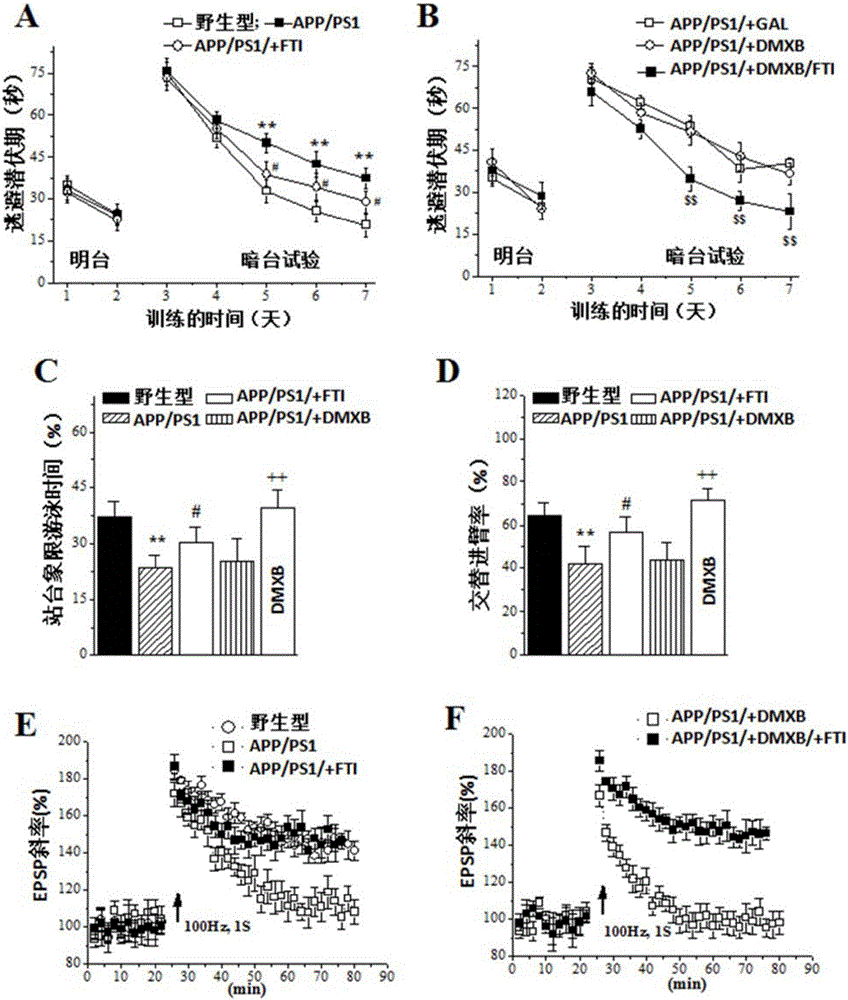
[0070] Example 3: Farnesyltransferase Inhibitor Improves Cognitive Function in Alzheimer's Disease Mice——Anti-Alzheimer's Disease Dementia (1)
[0071] Experimental main materials:
[0072] The animals were kept in the Experimental Animal Center of Nanjing Medical University, and maintained in an environment with a temperature of 23±2°C, a humidity of 55±5%, and a 12:12h light / dark cycle. They have free access to food and water.
[0073] Drugs and Reagents
[0074] With embodiment 1 and embodiment 2.
[0075] α7nACh receptor agonist 3-(2,4-dimethoxybenzylidene)-anabaseine (DMXB, provided by Dazhao Pharmaceutical Co., Ltd., Japan, code name GTS-21, Taisho Pharmaceuticals, Tokushima, Japan) was dissolved in normal saline for intraperitoneal injection (0.2mg / kg). The acetylcholinesterase inhibitor Galantamine hydrobromide (GAL, galantamine hydrobromide, 99%, Melonepharma, MD1560, Dalian, P.R. China) was dissolved in normal saline for intraperitoneal injection (2 mg / kg).
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com