Methods of treating cancer with farnesyltransferase inhibitors
A technology of farnesyltransferase and inhibitor, applied in the field of cancer therapy, can solve the problem of different response and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0389] Neutropenia predicts clinical response to tipifarnib in MDS
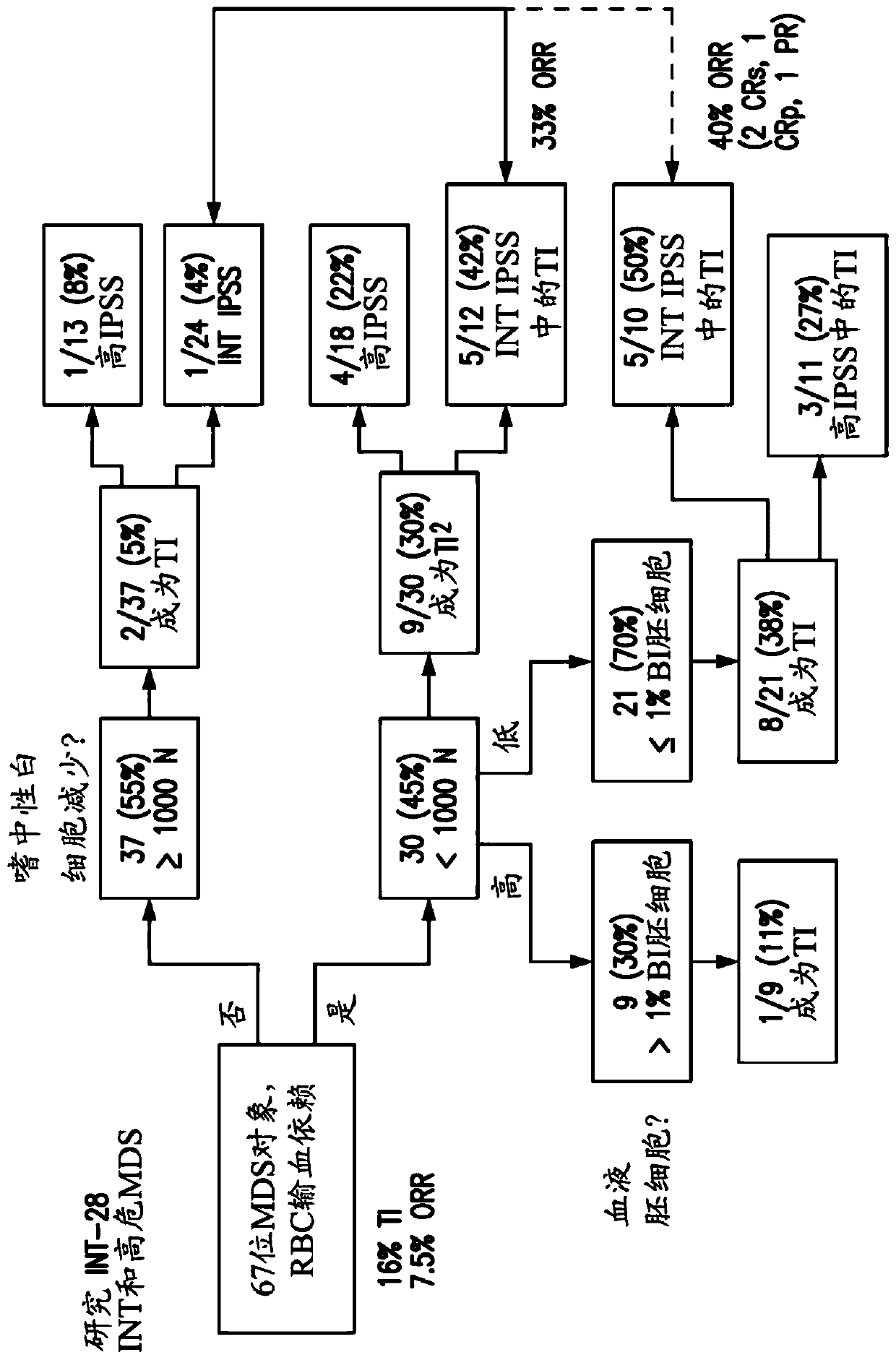
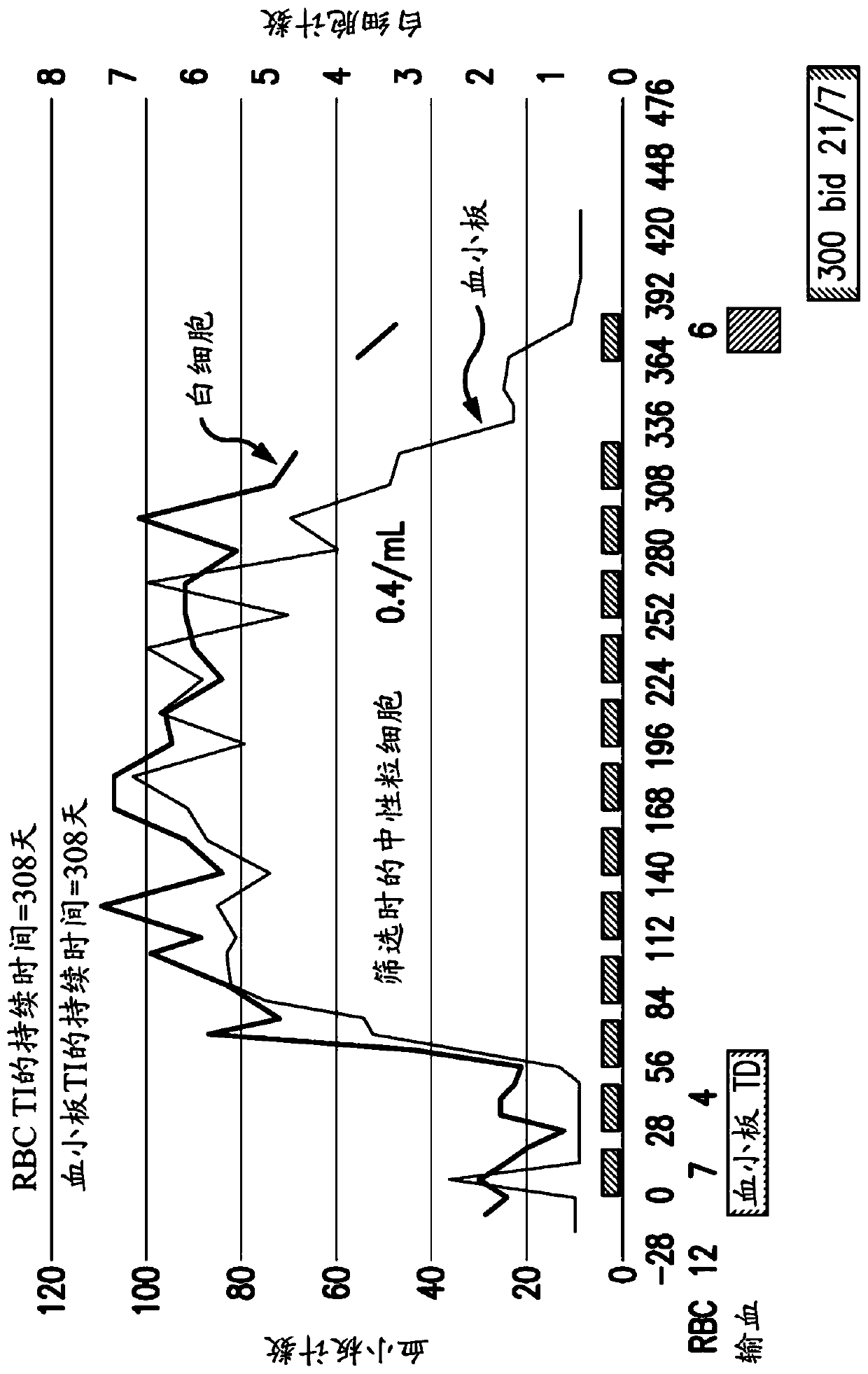
[0390] A clinical study of tipifarnib (300mg bid (twice a day), 3 weeks on / 1 week off) was conducted in 82 high-risk MDS / CMML subjects. At study entry, 67 subjects were transfusion dependent (TD), where subjects who received one or several red blood cell (RBC) transfusions during the screening period through the first administration of tipifarnib were classified as TD. Eleven (16%) of the initial 67 TD subjects receiving tipifarnib were found to be converted to transfusion independence (TI), which persisted from day 55 to day 666.
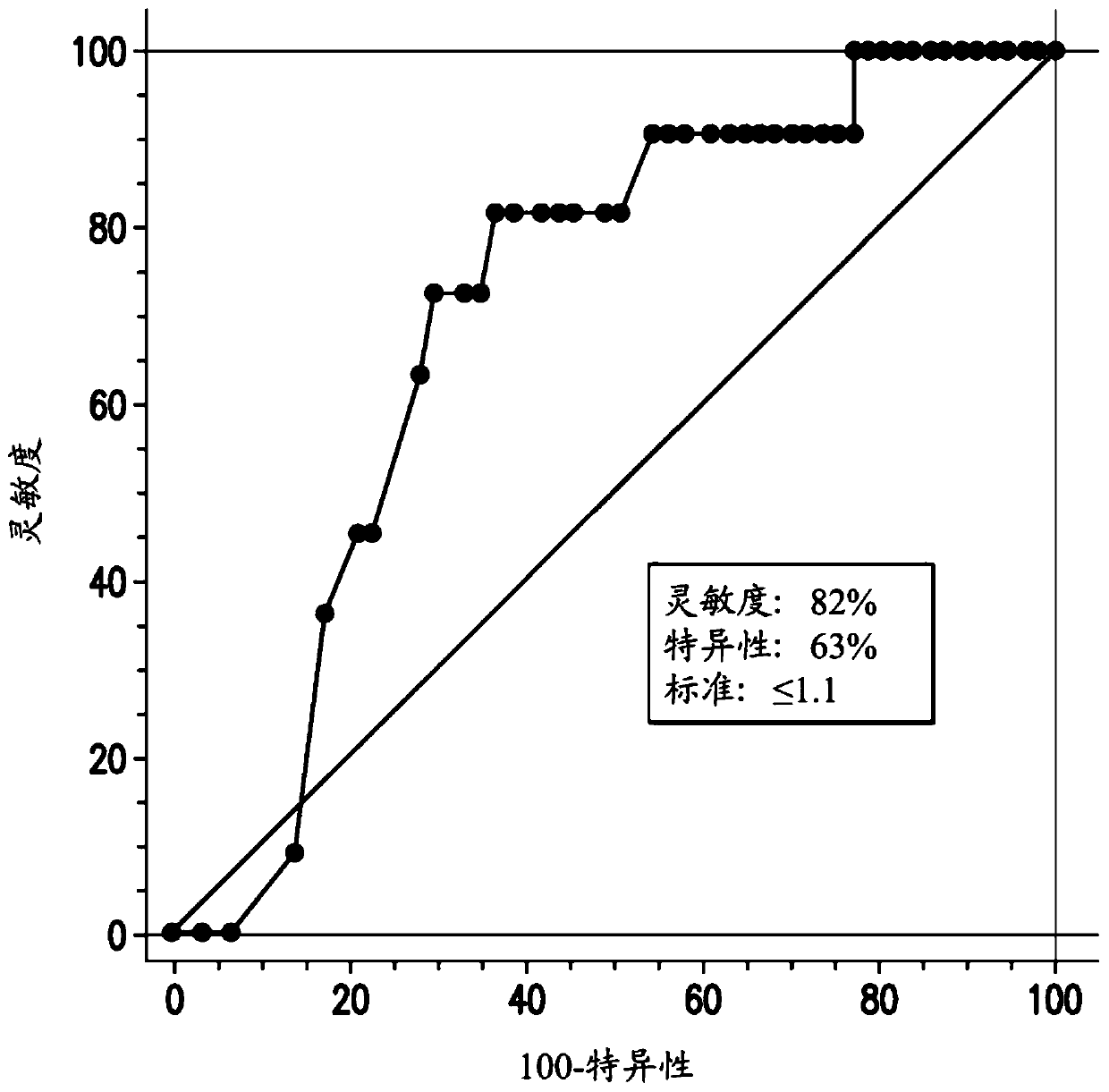
[0391] Figure 1A A graph showing the results of a retrospective analysis of the trial results for these 67 transfusion-dependent MDS subjects is shown. Initially, 30 / 67 (45%) MDS subjects were classified as having moderate to severe neutrophils based on their absolute neutrophil count (ANC) 1,000 cells / μl blood. During the tipifarnib study, 9 / 30 (30%) MDS subjects with moderat...
Embodiment II
[0396] Bone marrow homing of myeloid cells as indicated by neutropenia and / or low blood blast counts predicts clinical benefit of tipifarnib in AML
[0397] Clinical studies with tipifarnib were conducted in newly diagnosed elderly patients with high-risk AML (CTEP-20, phase 2) and patients with relapsed and refractory AML (INT-17, phase 2). Patient selection in these studies was not based on genetic markers. Anecdotal evidence of single-agent activity of tipirfarnib has been reported. However, overall clinical activity across the patient population does not support registration of tipifarnib. Additionally, a contemporaneous Ph3 study (AML301, N=457) failed to identify increased Tipirfarnib efficacy (hazard ratio = 1.02, not significant). In AML301, the median OS for the overall study was 107 days for the tipifarnib arm and 109 days for the BSC arm.
[0398] Figure 2A Graph showing the amount of bone marrow blasts, blood blasts and WBCs observed in samples from elderly p...
Embodiment III
[0410] CXCL12 expression or CXCL12 / CXCR4 expression ratio predicts clinical benefit of tipifarnib in multiple hematological malignancies
[0411] In the CTEP20 study, 9 complete responses (CR) and 13 best responses for disease progression (PD) were observed in 34 pts with available BM gene expression data. CXCL12 expression alone or as a ratio of expression to its receptor CXCR4 predicted complete response to tipifarnib treatment (p=0.08, p=0.001) ( Figure 20A ). CXCL12 and CXCL12 / CXCR4 ratio also indicated 2 partial responses (PR) observed in 13 highly advanced relapsed / refractory PTCL patients with available GEP (p=0.009, p=0.0007) ( Figure 20B ). Four objective responses and one tumor lysis syndrome were reported in 17 tipifarnib-treated CMML subjects with available pretreatment BM gene expression data ( Figure 20C ). CXCL12 expression (p=0.07) and CXCL12 / CXCR4 ratio (p=0.03) predicted those events ( Figure 20C ). In the INT-17 study, only 3 CRs were reported amon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com