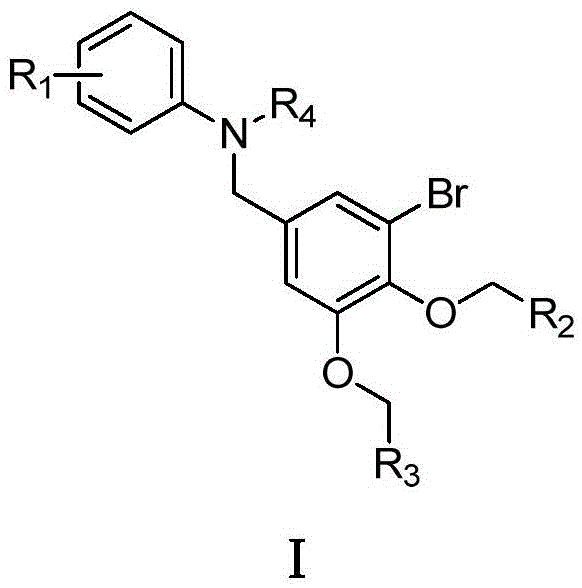
Benzyl-substituted aniline compounds and their applications
An aniline compound and compound technology are applied in the preparation of aminohydroxy compounds, the preparation of cyanide reaction, the preparation of organic compounds, etc., which can solve the problems of uncontrolled cell proliferation and the like, and achieve the effect of good drug prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
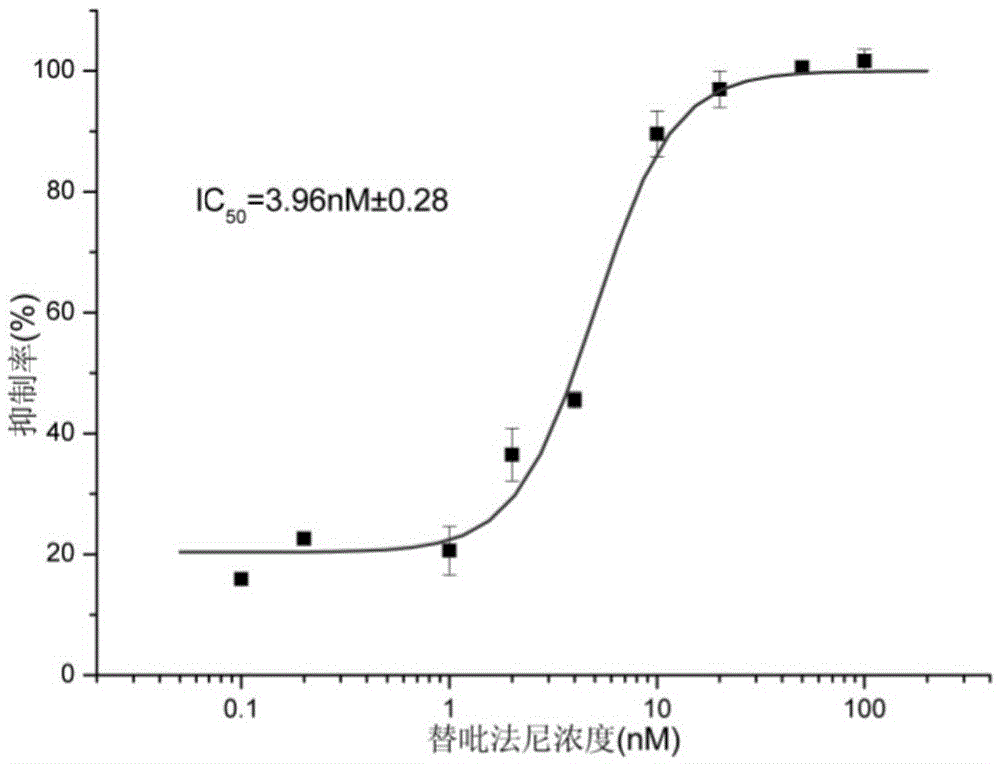
Image
Examples
Embodiment 1
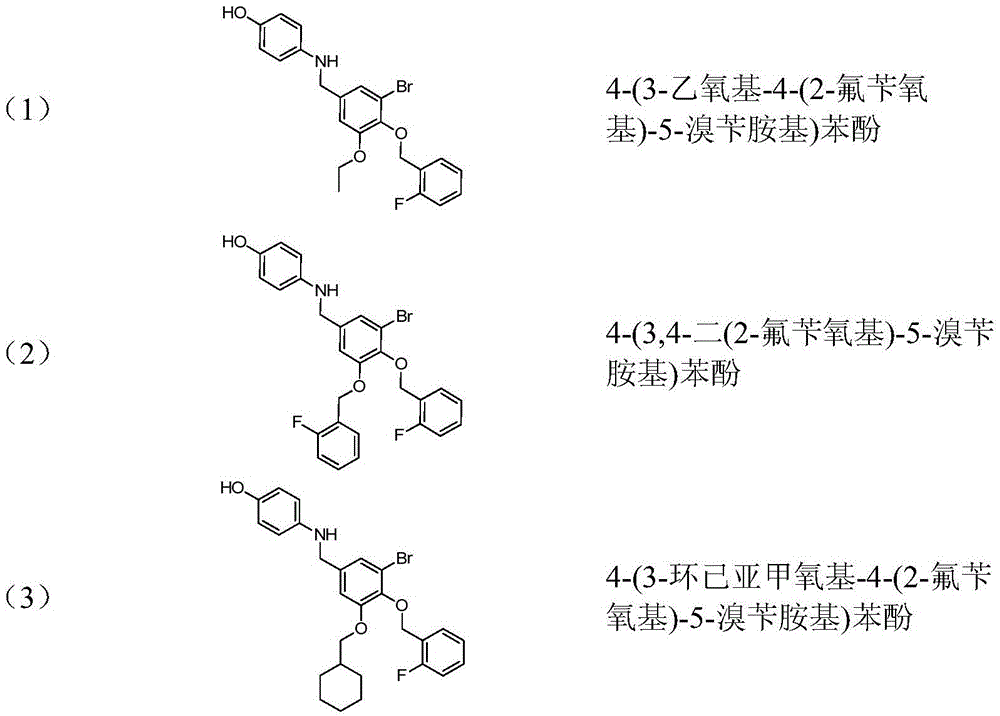
[0030] 4-(3-ethoxy-4-(2-fluorobenzyloxy)-5-bromobenzylamino)phenol (1)
[0031]
[0032] The synthetic route of compound 1 is as follows:
[0033]
[0034] Synthesis of 3-ethoxy-4-hydroxy-5-bromobenzaldehyde (1.1)
[0035] 3-Ethoxy-4-hydroxybenzaldehyde (6.64 g, 40.0 mmol) was dissolved in acetic acid (80 mL) and fully dissolved. Then, liquid bromine (2.46 mL) was added dropwise to the reaction solution. After the dropwise addition, the reaction was carried out at room temperature for 3 hours, and turbidity appeared. After the reaction was monitored by TLC, a solid was obtained by suction filtration, and the solid was recrystallized with 50% ethanol aqueous solution to obtain 7.8 g of the target compound 1.1 with a yield of 80%.
[0036] Synthesis of 3-ethoxy-4-(2-fluorobenzyloxy)-5-bromobenzaldehyde (1.2)
[0037] Compound 1.1 (3.0g, 12.3mmol) and o-fluorobenzyl bromide (2.31g, 12.3mmol) were dissolved in DMF (5mL), and potassium carbonate (2.04g, 14.75mmol) was adde...
Embodiment 2
[0044] 4-(3,4-bis(2-fluorobenzyloxy)-5-bromobenzylamino)phenol (2)
[0045]
[0046] The synthetic route of compound 2 is as follows:
[0047]
[0048] The synthesis of compound 2.1 is as shown in compound 1.1, the difference is that the 3-ethoxyl-4-hydroxybenzaldehyde used in the process of synthesis 1.1 is replaced by 3,4-dihydroxybenzaldehyde; the synthesis of compound 2.2 is as in compound 1.2, the difference is that compound 1.1 is replaced by compound 2.1; the synthesis of compound 2 is as shown in compound 1, the difference is that compound 1.2 is replaced by compound 2.2.
[0049] 1 H NMR (400MHz, DMSO-d 6 ): δ8.43(s, 1H), 7.59-6.44(m, 10H), 6.53(d, 2H, J=8.4Hz), 6.45(d, 2H, J=8.4Hz), 5.66(t, 1H, J=6.0Hz), 5.20(s, 2H), 4.99(s, 2H), 4.15(d, 2H, J=6.0Hz).
[0050] HRMS (ESI) calcd for C 27 h 22 BrF 2 NO 3 [M+H] + 526.0829, found 526.0834.
Embodiment 3
[0052] 4-(3-Cyclohexamethyleneoxy-4-(2-fluorobenzyloxy)-5-bromobenzylamino)phenol (3)
[0053]
[0054] The synthetic route of compound 3 is as follows:
[0055]
[0056] Synthesis of Compound 3.1:
[0057] Compound 2.1 (300 mg, 1.39 mmol) was dissolved in DMF (3.0 mL), and sodium bicarbonate (174 mg, 2.07 mmol) and potassium iodide (69 mg, 0.414 mmol) were added, and o-fluorobenzyl bromide (519 mg, 2.76mmol), magnetically stirred, reacted overnight at 40°C, and followed the conversion of raw materials by TLC. The crude product was dissolved in water and extracted with ethyl acetate. Combined organic phases, anhydrous MgSO 4 Dry and concentrate. The product was separated by silica gel column chromatography (DCM / PE=9 / 1, v / v) to obtain 270 mg of the product as a white solid, with a yield of 60%.
[0058] The synthesis conditions of compound 3.2 are as the synthesis conditions of compound 1.2, and the difference is that two different reactants are used, namely compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com