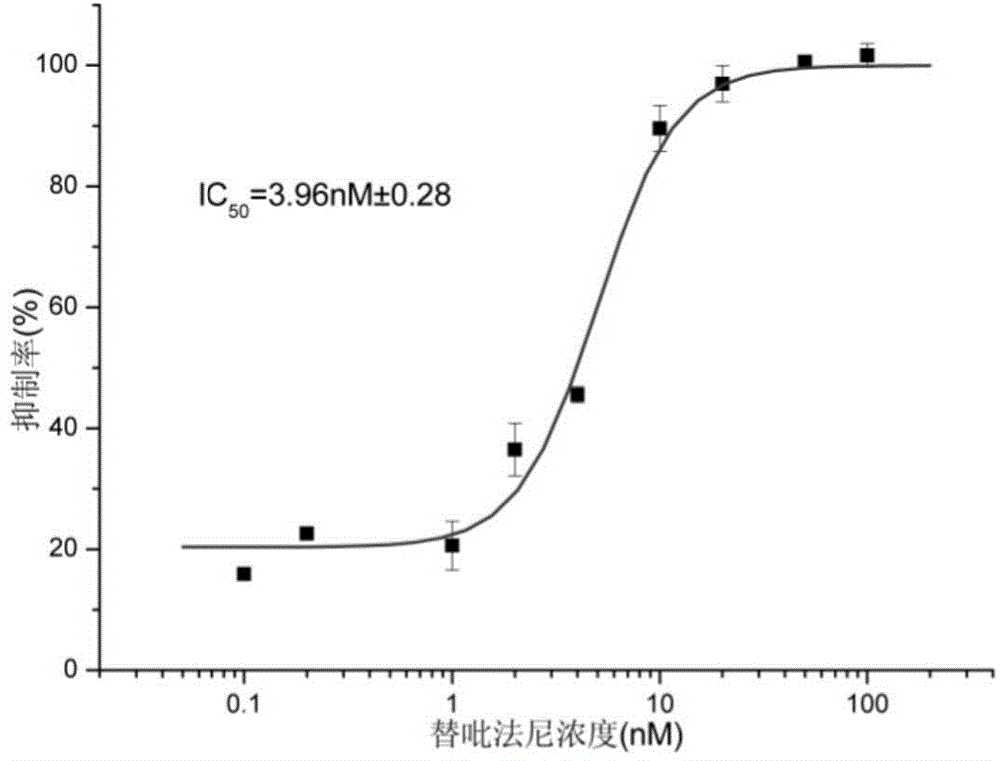
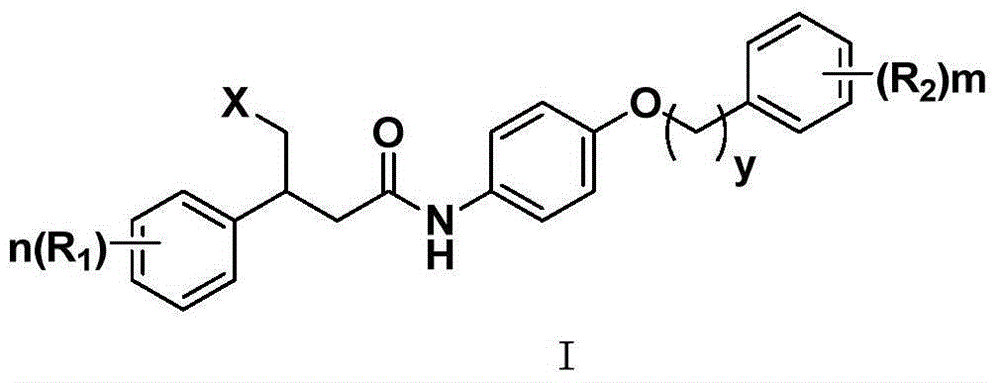
Aniline compound as farnesyltransferase inhibitor and application thereof
A technology of aniline compounds and farnesyltransferase, which is applied in the field of aniline compounds, can solve problems such as uncontrolled cell proliferation and achieve good drug prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
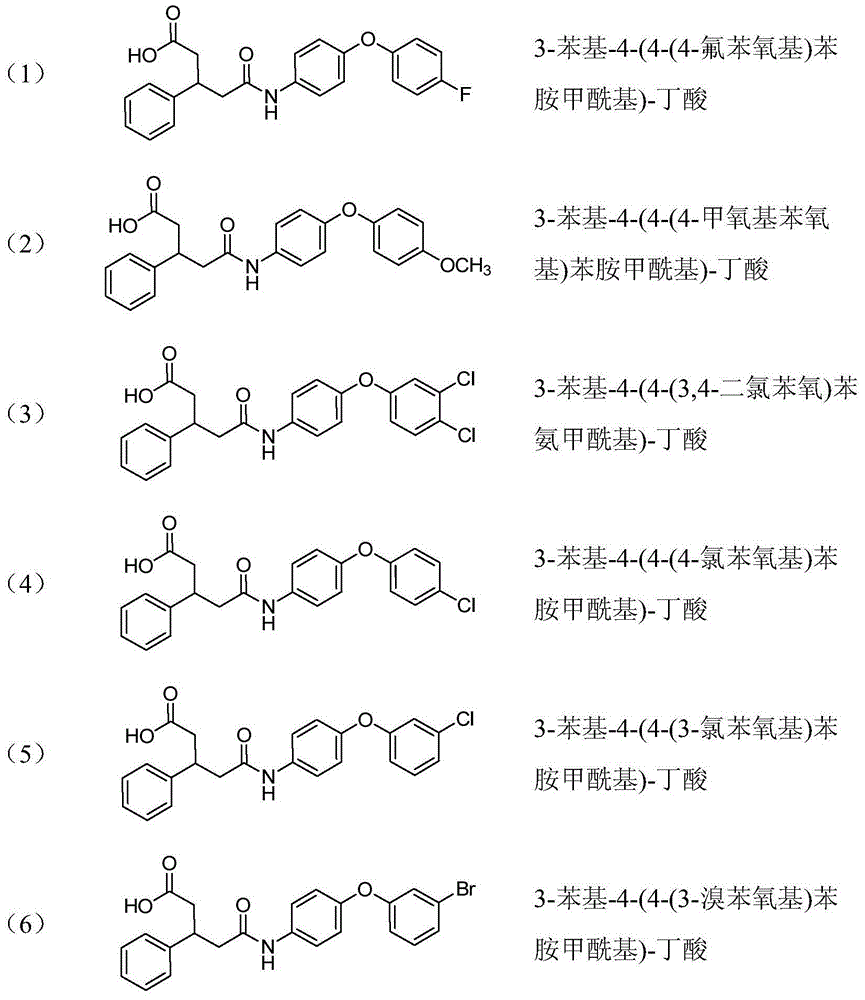
[0030] 3-Phenyl-4-(4-(4-fluorophenoxy)anilinoyl)-butyric acid (1)
[0031]
[0032] Synthesis of 4-(4-fluorophenoxy)aniline (1.4)
[0033]
[0034] p-Fluoronitrobenzene (705 mg, 5.0 mmol), p-fluorophenol (560 mg, 5.0 mmol) and potassium carbonate (2.07 g, 15.0 mmol) were dissolved in DMF (5 mL). The reaction was warmed to 70°C and stirred overnight. After the reaction was monitored by TLC, the reaction solution was dissolved in ethyl acetate, extracted with water and brine, the organic phase was separated and dried with anhydrous sodium sulfate, and the organic solvent was removed under reduced pressure to obtain 990 mg of light yellow product 1.3, with a yield of 85%.
[0035] 1 H NMR (400MHz, DMSO-d 6 ): δ8.26(d, 2H, J=8.8Hz), 7.37-7.11(m, 4H), 7.12(d, 2H, J=9.2Hz).
[0036] SnCl 2 ·H 2 O (2.25g, 10mmol) was dissolved in concentrated hydrochloric acid (10mL), then compound 1.3 was added into the mixture (249mg, 1mmol), and the reaction solution was heated to refl...
Embodiment 2
[0049] 3-Phenyl-4-(4-(4-methoxyphenoxy)anicarboyl)-butyric acid (2)
[0050]
[0051] For the synthesis method, refer to Example 1, except that the compound 1.2 used in the synthesis of compound 1.4 was replaced with p-methoxyphenol, and the product was a white solid with a yield of 61%.
[0052] 1 H NMR (400MHz, DMSO-d 6 ): δ12.05(s, 1H), 9.82(s, 1H), 7.47(d, 2H, J=8.8Hz), 7.29-7.27(m, 4H), 7.21-7.15(m, 1H), 6.93( s, 4H), 6.85 (d, 2H, 9.2Hz), 3.72-3.53 (m, 1H), 2.71-2.54 (m, 4H).
[0053] 13 C NMR (100MHz, DMSO-d 6 ): δ173.3, 169.6, 155.8, 153.6, 150.7, 144.1, 134.7, 128.7, 127.9, 126.8, 121.3, 120.4, 118.4, 115.5, 55.9, 43.2, 40.6, 38.7.
[0054] HRMS (ESI) calcd for C 24 h 22 NO 5 [M-H] + 404.1498, found 404.1502.
Embodiment 3
[0056] 3-Phenyl-4-(4-(3,4-dichlorophenoxy)phenylcarbamoyl)-butanoic acid (3)
[0057]
[0058] For the synthesis method, refer to Example 1, except that the compound 1.2 used in the synthesis of compound 1.4 was replaced with 3,4-dichlorophenol, and the product was a white solid with a yield of 45%.
[0059] 1 H NMR (400MHz, DMSO-d 6 ): δ12.07(s, 1H), 9.95(s, 1H), 7.60-7.56(m, 3H), 7.30-7.29(m, 4H), 7.21-7.19(m, 2H), 7.03(d, 2H , J=8.8Hz), 6.94(d, 1H, J=8.4Hz), 3.65-3.55(m, 1H), 2.73-2.55(m, 4H).
[0060] 13 C NMR (100MHz, DMSO-d 6 ): δ173.3, 169.8, 157.7, 150.8, 144.0, 136.4, 132.4, 131.9, 128.7, 127.9, 126.9, 125.2, 121.3, 120.5, 119.8, 118.3, 43.2, 38.7.
[0061] HRMS (ESI) calcd for C 23 h 20 ClNO 4 [M+H] + 444.0769, found 444.0778.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com