Iminosugar in crystalline form
a technology of iminosugar and crystalline form, which is applied in the direction of aminosugars, biocide, plant growth regulators, etc., can solve the problem of insufficient pureness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of N-butyl 2,3,4,6-tetra-O-benzyl-1,5-dideoxy-1,5-imino-D-glucitol (III)
[0069]A solution of oxalyl chloride (99.8 g, 0.79 mol) in dichloromethane (300 mL) is cooled to −75° C., treated under inert atmosphere in sequence with a solution of DMSO (77.1 g, 0.99 mol) in dichloromethane (100 ml) added by slow dripping, and then, after about 1 h, with a solution obtained by dissolving 2,3,4,6-tetra-O-benzyl-D-glucitol, prepared as in Synthesis 1999, 571-573 (HPLC assay 94.5%, 105.9 g, 0.18 mol) in dichloromethane (100 mL), added by slow dripping. The reaction mixture is maintained under stirring at a temperature not exceeding 65° C., and treated after about 2 hours with triethylamine (187 g, 1.85 mol), added by slow dripping, maintaining the reaction mixture under stirring at a temperature not exceeding 50° C. for at least 4 hours. The end-of-reaction mixture is then added to a mixture maintained under stirring in an inert atmosphere at the temperature of 0° C., obtained by mixin...
example 2
Crystallisation of N-butyl 2,3,4,6-tetra-O-benzyl-1,5-dideoxy-1,5-imino-D-glucitol (III)
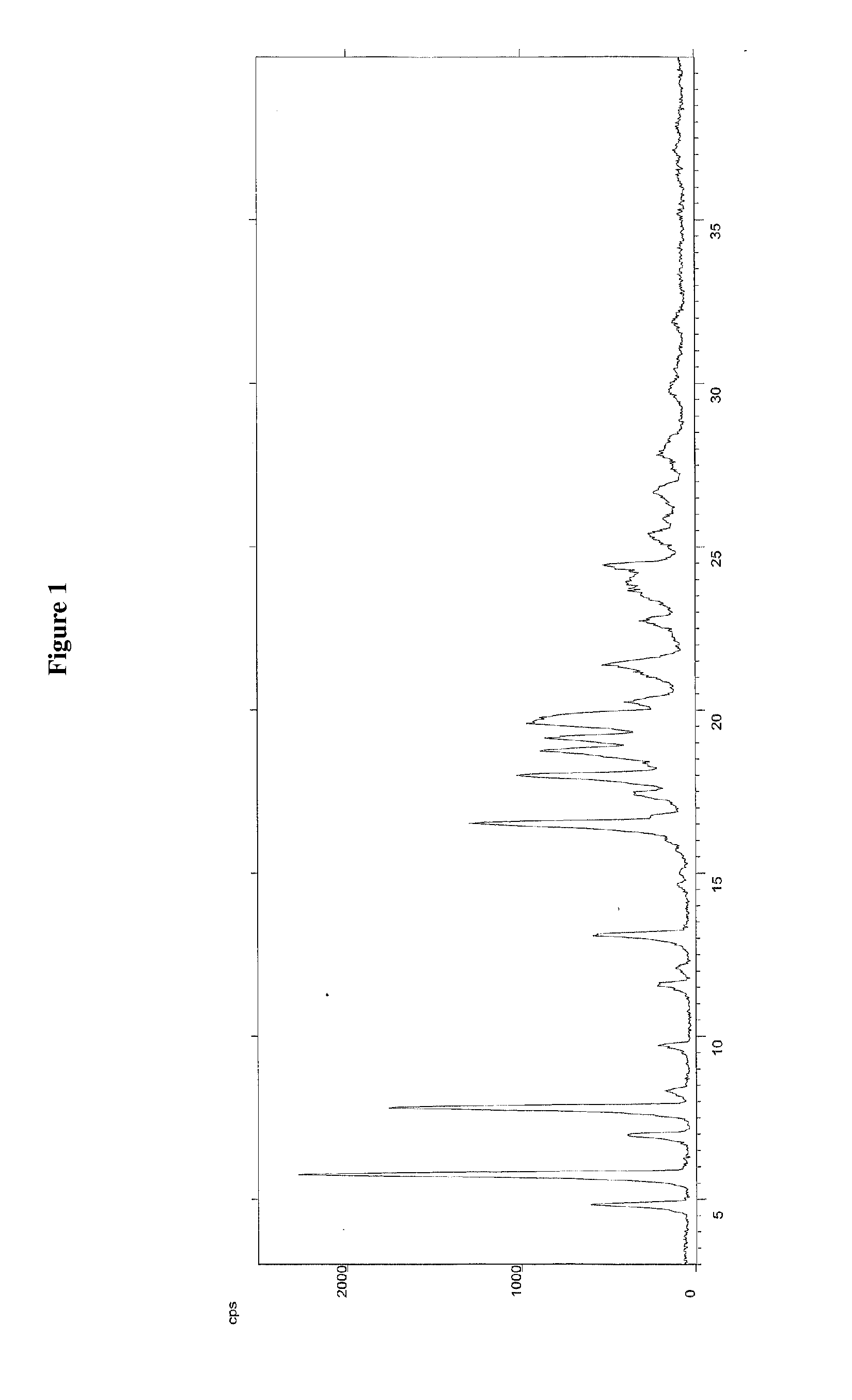
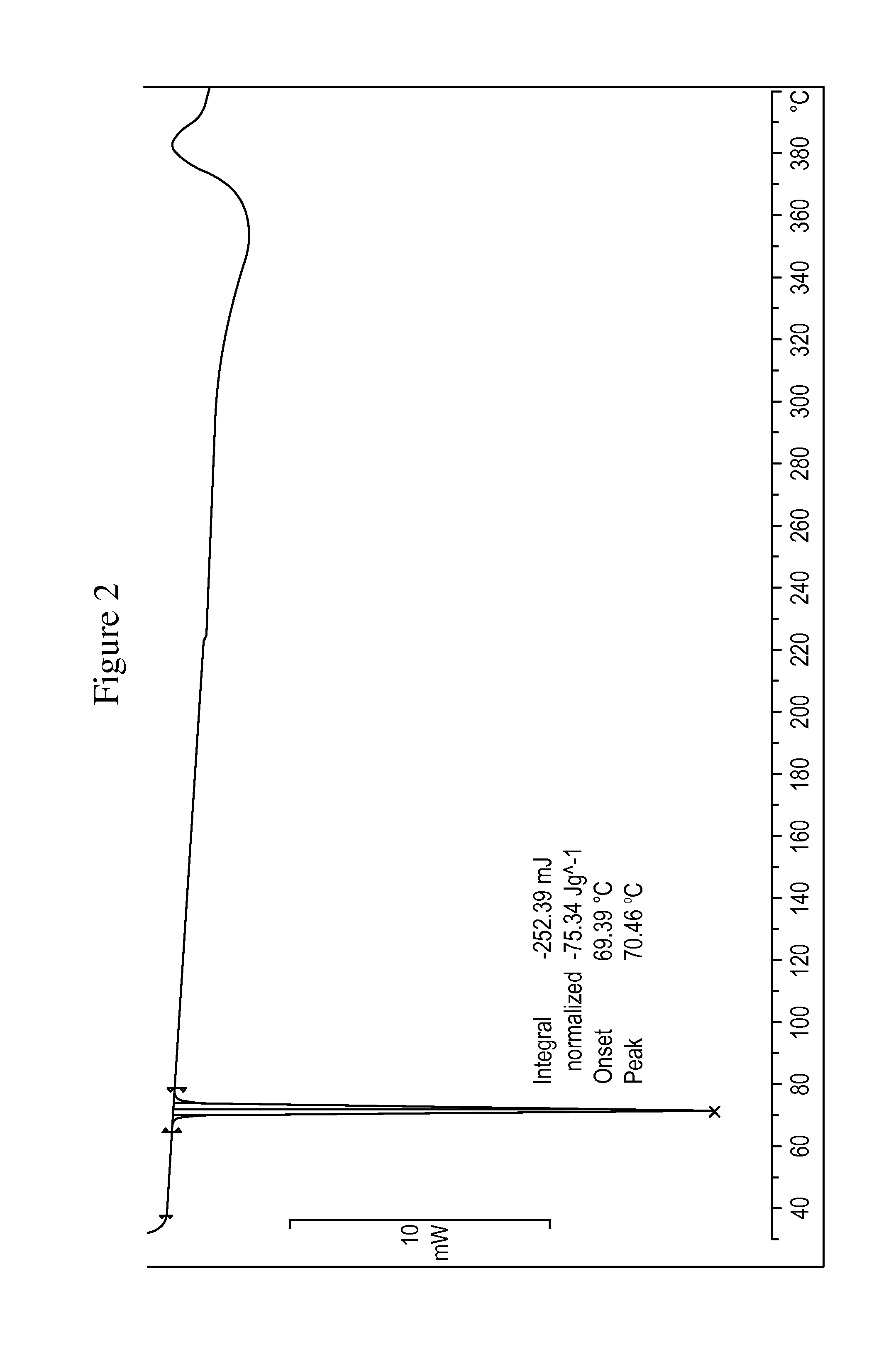
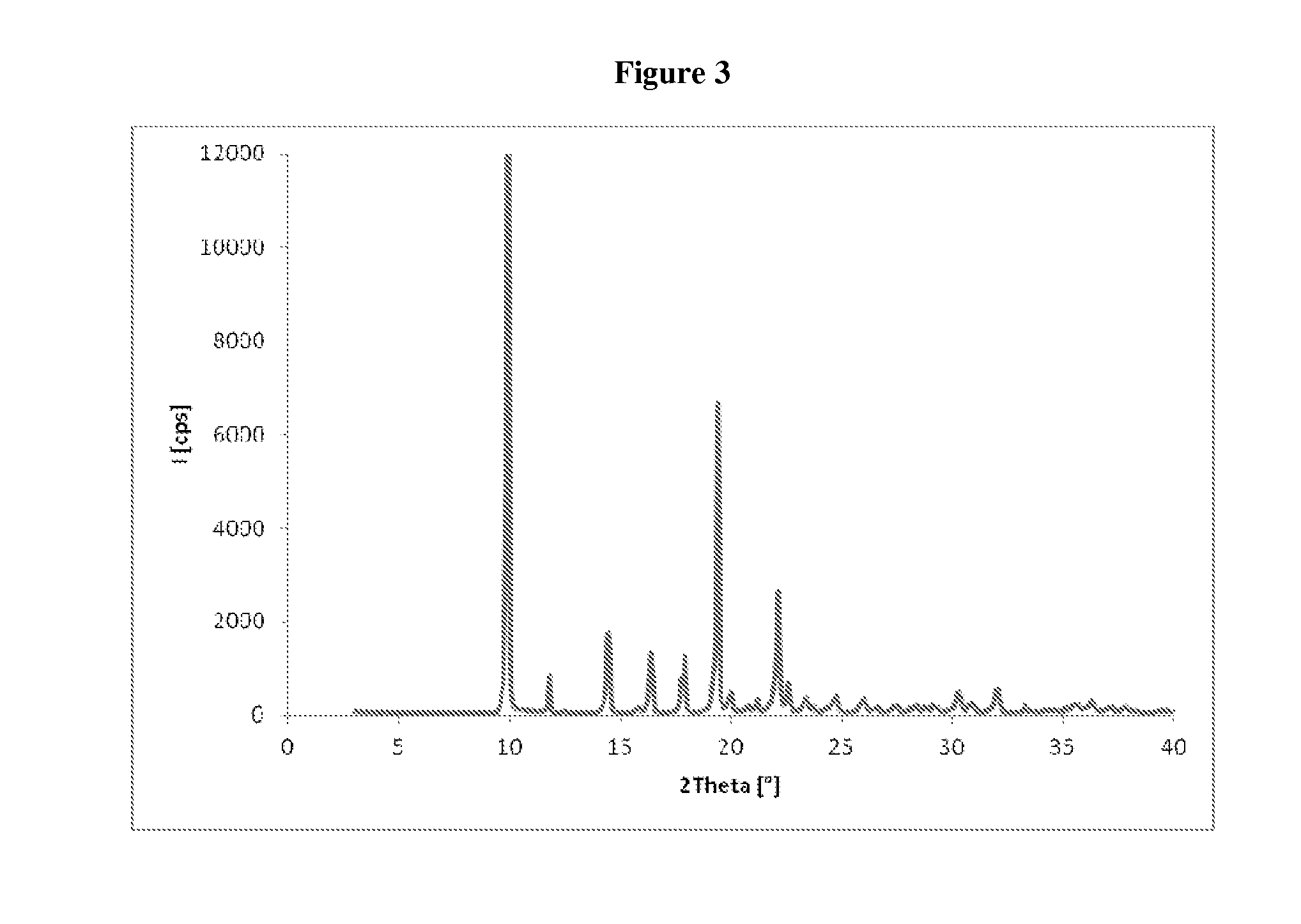
[0070]The crude compound of formula (III), obtained as in Example 1, is dissolved in isopropanol (120 mL), and the resulting solution is cooled in an ice bath and treated with water (18 mL). The suspension obtained is maintained under stirring at about 20° C. for 15 hours, and then filtered through a Büchner funnel and the panel washed with isopropanol. The wet solid is stove-dried at the temperature of 50° C., under vacuum, to a constant weight, supplying 50 g of compound of formula (III) with high chemical purity, in crystalline form A, wherein the main peaks (expressed in 2θ°) are found at 4.83, 5.76, 6.96, 7.80, 13.08, 16.50, 17.97, 18.75, 19.14, 19.62. Said crystalline product presents a DSC thermogram as illustrated in FIG. 2, and a water content below 0.1%.
[0071]The compound of formula (III) can be recrystallised from isopropanol alone to obtain a compound of formula (III) with a purity, c...
example 3
Synthesis of Miglustat of Formula (I)
[0072]A solution obtained by mixing N-butyl 2,3,4,6-tetra-O-benzyl-1,5-dideoxy-1,5-imino-D-glucitol of formula (III), obtained as in Example 2 (105.1 g, 0.17 mol) in methanol (500 mL) in the presence of 32% HCl (43.5 g), is treated with 16% Pd / C (10.2 g). The mixture is maintained under vigorous stirring under hydrogen atmosphere at 4 bars for about 4 hours, and then filtered through a perlite panel, and the solution obtained is concentrated at low pressure. The solid residue thus obtained is dissolved in water (100 mL), and the acid solution obtained is passed through a column on an ion-exchange resin activated in basic form (Amberlite IRA 900Cl). The fractions that tested positive to the ninhydrin assay were combined and concentrated at low pressure, obtaining 50 g of miglustat as an oily residue, having a chemical purity exceeding 98%, calculated by HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com