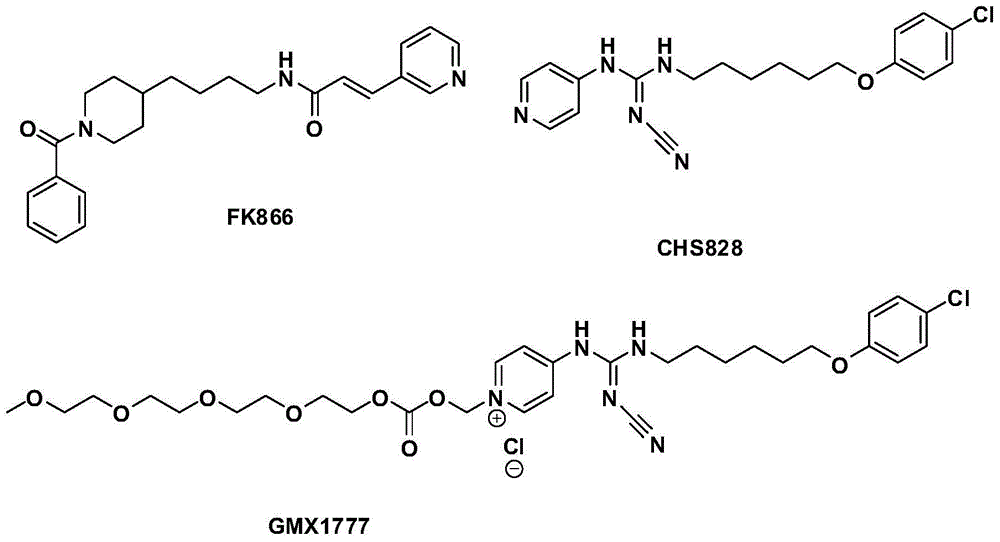
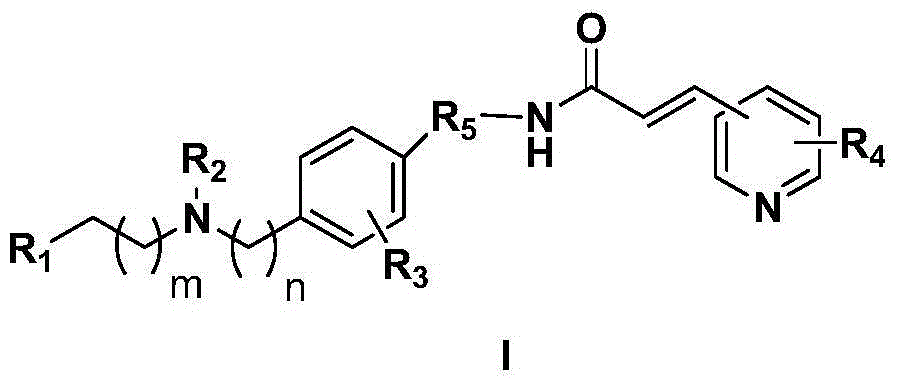
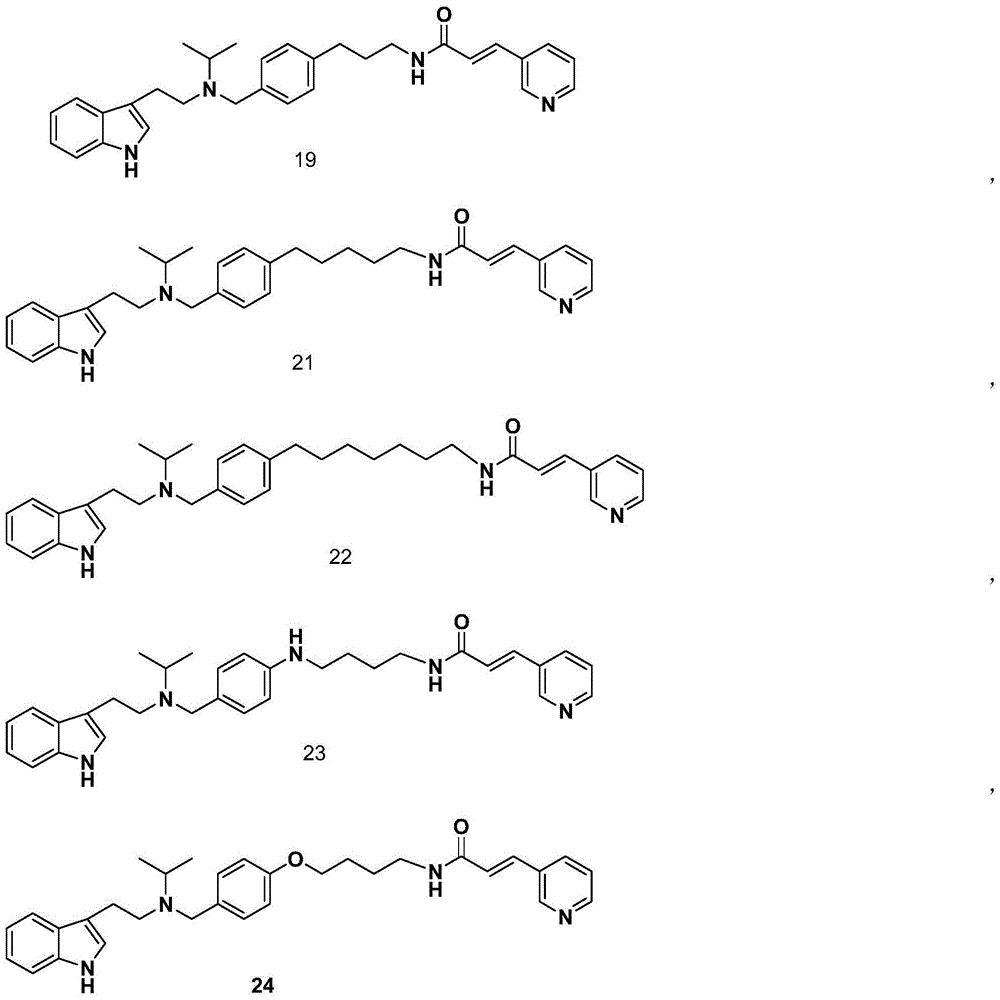
Novel nicotinamide ribose phosphate transferase inhibitor as well as synthetic method and application thereof
A technology of phosphoribose and transferase, applied in the field of medicine, to achieve the effect of simple operation, excellent anti-tumor activity, and cheap synthetic raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]
[0046] Compound 9 (408mg, 1mmol) was dissolved in anhydrous DCM, triethylamine (0.4mL, 3mmol) was added at 0°C, then methanesulfonyl chloride (0.12mL, 1.5mmol) was slowly added dropwise, and the reaction was completed after the dropwise addition was completed. After 1 hour, add saturated sodium bicarbonate solution to quench the reaction, add DCM to dilute, then wash with saturated sodium bicarbonate and saturated brine, then dry over anhydrous magnesium sulfate, filter, and concentrate to obtain a crude product that is directly used in the next reaction.
[0047] Dissolve the crude product obtained in the previous step in DMF, add sodium azide (195mg, 3mmol), react at 80°C for 2 hours, cool to room temperature, spin off DMF under reduced pressure, add diethyl ether for extraction, and use saturated ammonium chloride for the organic phase , washed with saturated brine, then dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain a crude product ...
Embodiment 2
[0051]
[0052]Compound 10 (18mg, 0.12mmol) was dissolved in DMF, then EDCI (38mg, 0.2mmol), HOBt (20mg, 0.15mmol), TEA (0.03mL, 0.2mmol) were added successively, and finally compound 3 (41mg, 0.1 mmol), the reaction was stirred at room temperature for 5 hours, and the solvent was removed by rotary evaporation, then extracted with ethyl acetate, the organic phase was washed with saturated sodium bicarbonate and saturated brine successively, then dried over anhydrous magnesium sulfate, filtered, and the column layer was concentrated Compound 11 (46 mg, 86%) was obtained as a yellow solid.
[0053] 1 H NMR (400MHz, CDCl 3 ):δ8.72(s,1H),8.55-8.53(m,1H),7.77-7.73(m,1H),7.58(d,J=16.0Hz,1H),7.33(d,J=8.0Hz, 2H),7.30-7.16(m,1H),7.15(d,J=8.0Hz,2H),6.45(d,J=16.0Hz,1H),5.98(s,1H),5.33(s,1H), 3,39-3.32(m,2H),3.31(s,6H),2.60(t,J=8.0Hz,2H),1.67-1.55(m,4H),1.41-1.35(m,2H)ppm.MS (ESI,m / z):369(M + +1).
Embodiment 3
[0055]
[0056] Dissolve compound 11 (40mg, 0.07mmol) in ethyl acetate, then slowly add 3mol / L HCl (0.1mL, 0.3mmol) dropwise, react at room temperature for 12 hours, add saturated sodium bicarbonate solution dropwise to adjust the pH of the reaction solution When the value reached 7, ethyl acetate was added to dilute, the organic phase was washed with saturated sodium bicarbonate and saturated brine successively, then dried over anhydrous magnesium sulfate, filtered, and concentrated by column chromatography to obtain compound 12 (28 mg, 90%). 1 H NMR (400MHz, CDCl 3 ):δ9.94(s,1H),8.71(d,J=0.8Hz,1H),8.54-8.53(m,1H),7.78-7.73(m,3H),7.58(d,J=15.6Hz, 1H), 7.31-7.27(m, 2H), 6.47(d, J=15.6Hz, 1H), 6.08(s, 1H), 3.40-3.35(m, 2H), 2.68(t, J=8.0Hz, 2H ),1.71-1.57(m,4H),1.43-1.37(m,2H)ppm. 13 C NMR (125MHz, CDCl 3 ): δ191.9, 165.1, 150.2, 149.9, 149.0, 137.1, 134.4, 134.3, 130.7, 129.9, 129.0, 123.7, 122.9, 39.6, 35.9, 30.5, 29.4, 26.4ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com