Methods of treating cancer patients with farnesyl transferase inhibitors
A farnesyltransferase and inhibitor technology, applied in the fields of molecular biology and cancer biology, can solve problems such as different responses of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
[0752] Identification of immunological biomarkers related to the clinical benefit of tipifarnib
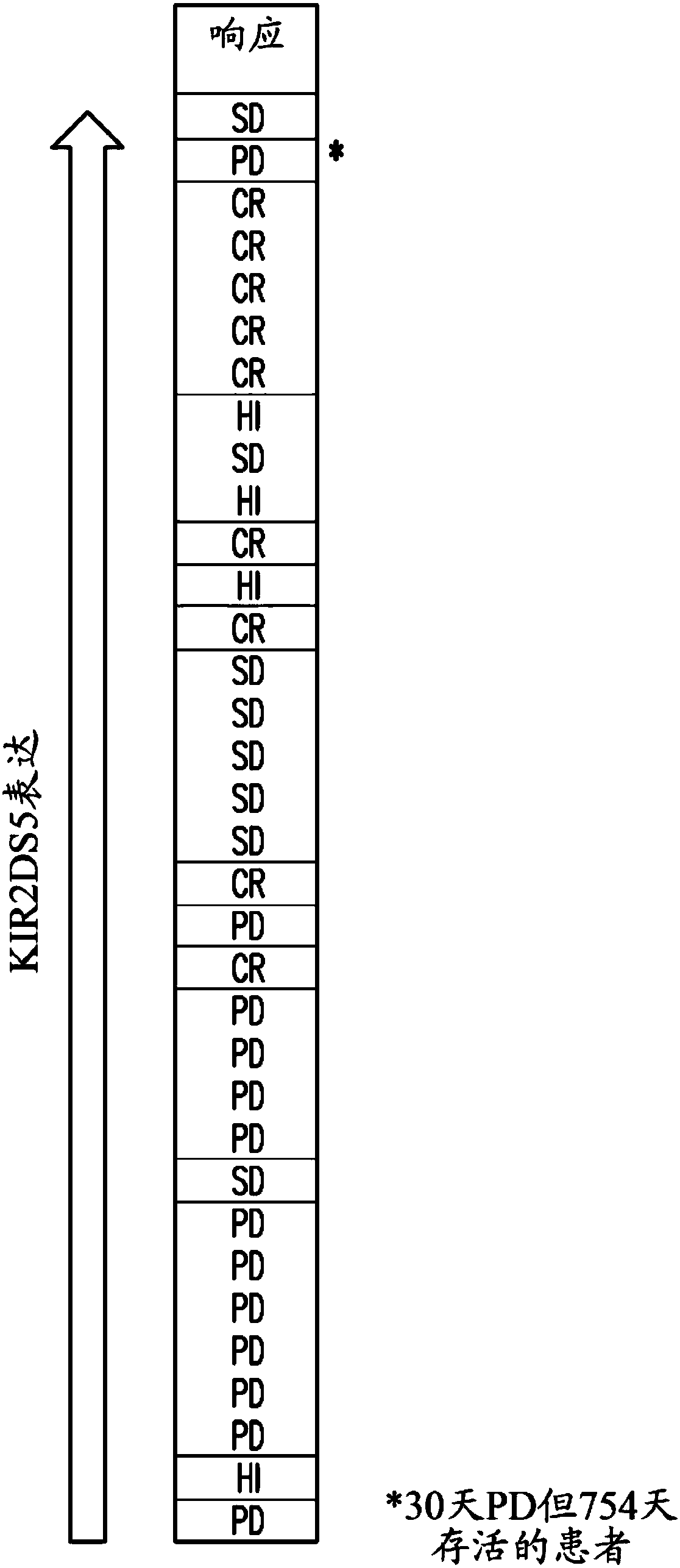
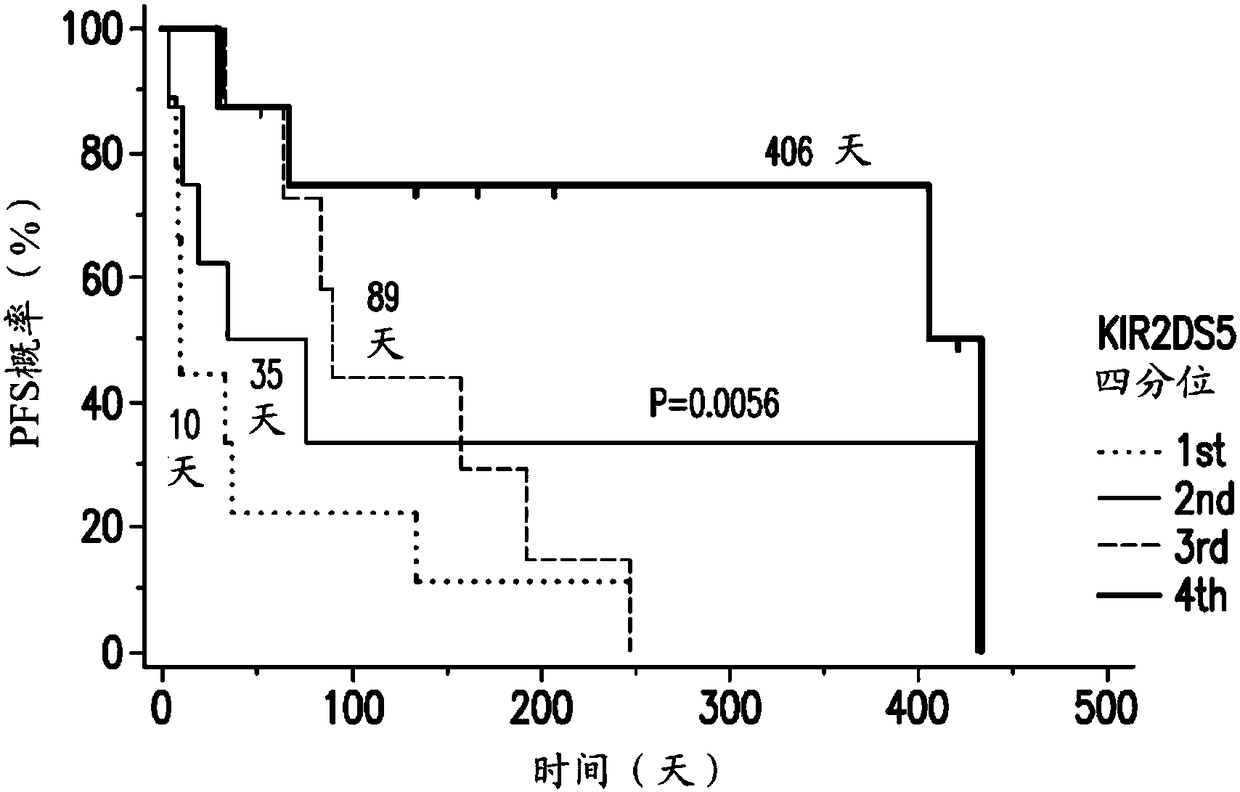
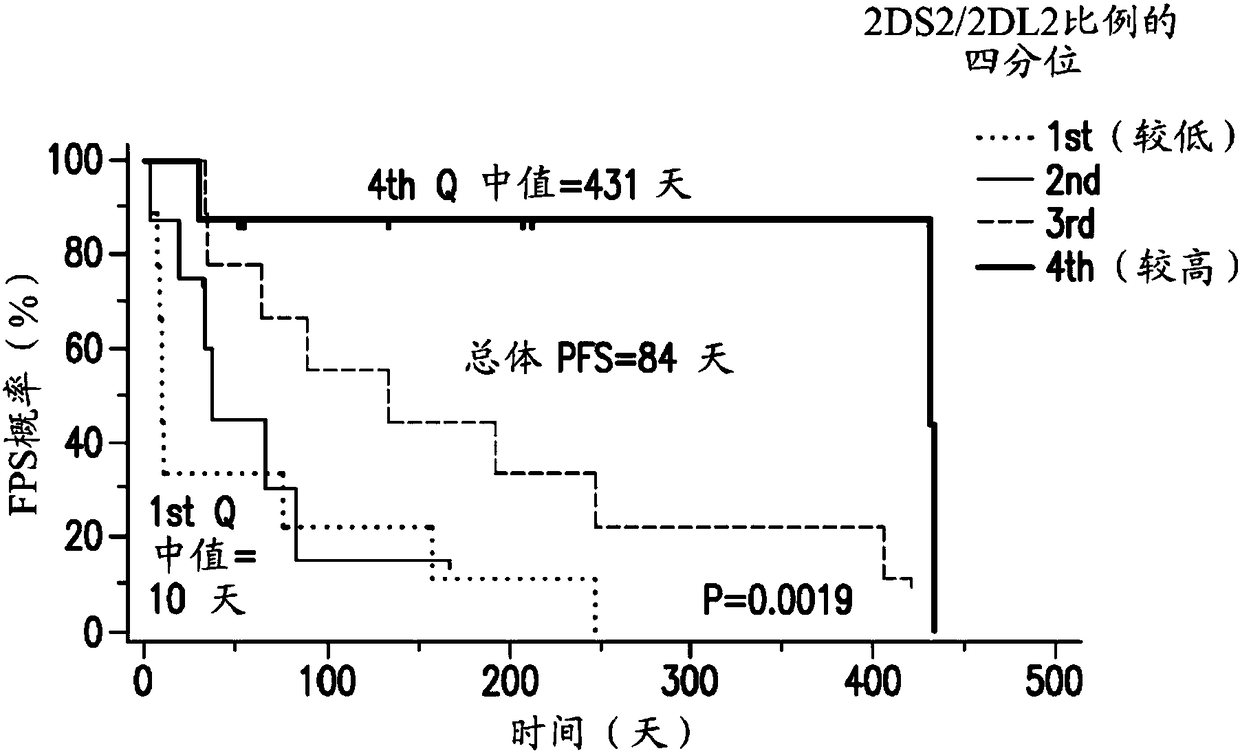
[0753] The analysis of gene expression distribution in bone marrow samples from two AML studies and the IC50 data of tipifarnib in multiple cell lines showed that a variety of immune-related genes, especially NK cell-related genes, are better than tipifarnib. Prognosis is relevant. Although some of these genes do not appear to be specific for tipifarnib treatment, other genes including KIR2DS2, KIR2DL2, KIR2DS5, KIR2DL5, and GZMM are specifically related to the clinical benefit of tipifarnib and not with other non-FTI chemotherapy Reagents such as cytarabine and mitoxantrone are related.
[0754] The current study used microarray data generated by global gene expression analysis of bone marrow samples collected in two clinical studies investigating the efficacy and safety of FTI Tipifarnib. One clinical study was conducted in adult patients over 65 years of age with previously untre...
Embodiment II
[0779] A. Stratification of AML patients in clinical trials of Tipifarnib.
[0780] Clinical trials can be conducted, which include KIR classification as part of the patient inclusion indicators. For example, research on the treatment of tipifarnib can be conducted in AML patients who are older than 60 years of age or who are not suitable for standard chemotherapy, or who have refractory or relapsed AML.
[0781] Before the AML patient enters the clinical trial, a bone marrow sample or blood sample is obtained from the patient. The sample is then subjected to microarray analysis. DNA was isolated from Trizol-treated bone marrow samples according to the manufacturer's instructions (Qiagen). Analyze the global gene expression of samples and quantitative polymerase chain reaction (QPCR) of specific genes, including KIR2DS2, KIR2DL2, KIR2DS5 and KIR2DL5. In addition, microarray analysis provides the genotype of the patient's KIR gene. If the patient is identified as a carrier of t...
Embodiment III
[0801] Individualized treatment judgment for CMML patients
[0802] The following process can be used to determine whether CMML patients are suitable for FTI treatment, for example, tipifarnib treatment.
[0803] Samples of BM were collected from patients before treatment, and monocytes were processed in situ. Total RNA was extracted from cell samples using Trizol kit (Qiagen, Santa Clarita, CA). The RNA quality was determined by evaluating the presence of ribosomal bands on an Agilent Bioanalyzer (Agilent, Palo Alto, CA). Good quality samples are further processed for microarray analysis.
[0804] For each sample, 1 g of total RNA (determined by OD260) was reverse transcribed using High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. The sample can then be incubated at 25°C for 10 minutes and then at 37°C for 30 minutes for optimal RNA conversion. QPCR was performed using ABI Prism 7900HT Sequence Detec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com