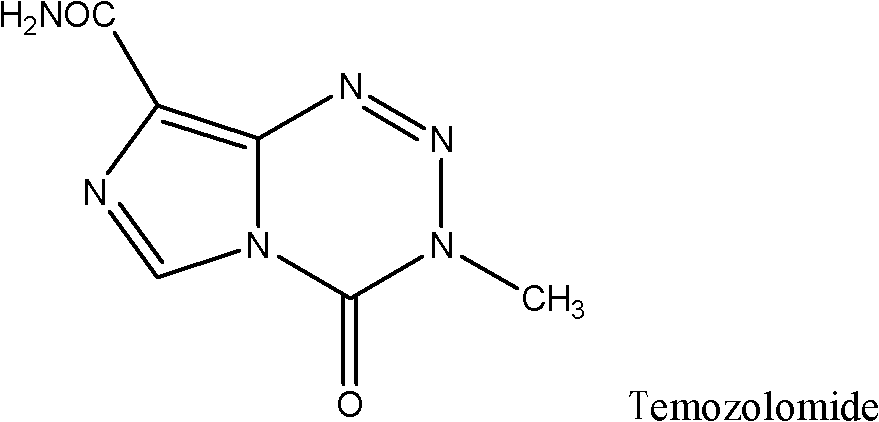
Method preparing temozolomide in one-pot mode and refining method of temozolomide
A technology of temozolomide and acid-binding agent, which is applied in the field of refining temozolomide, can solve the problems of reduced yield of final product, slow mass and heat transfer rate, complicated process route, etc., so as to avoid difficulties in transportation and storage, high yield and high Purity, the effect of avoiding high risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Example 1 One-pot method for preparing Temozolomide
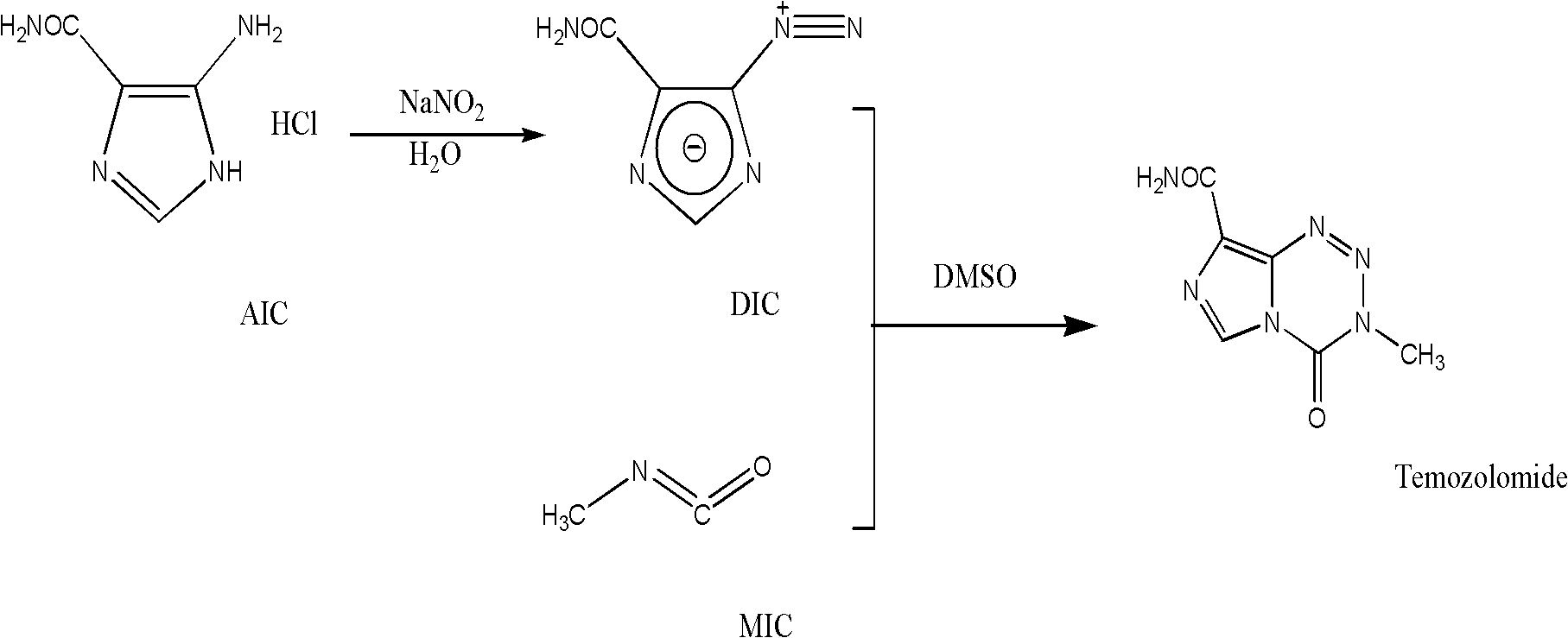
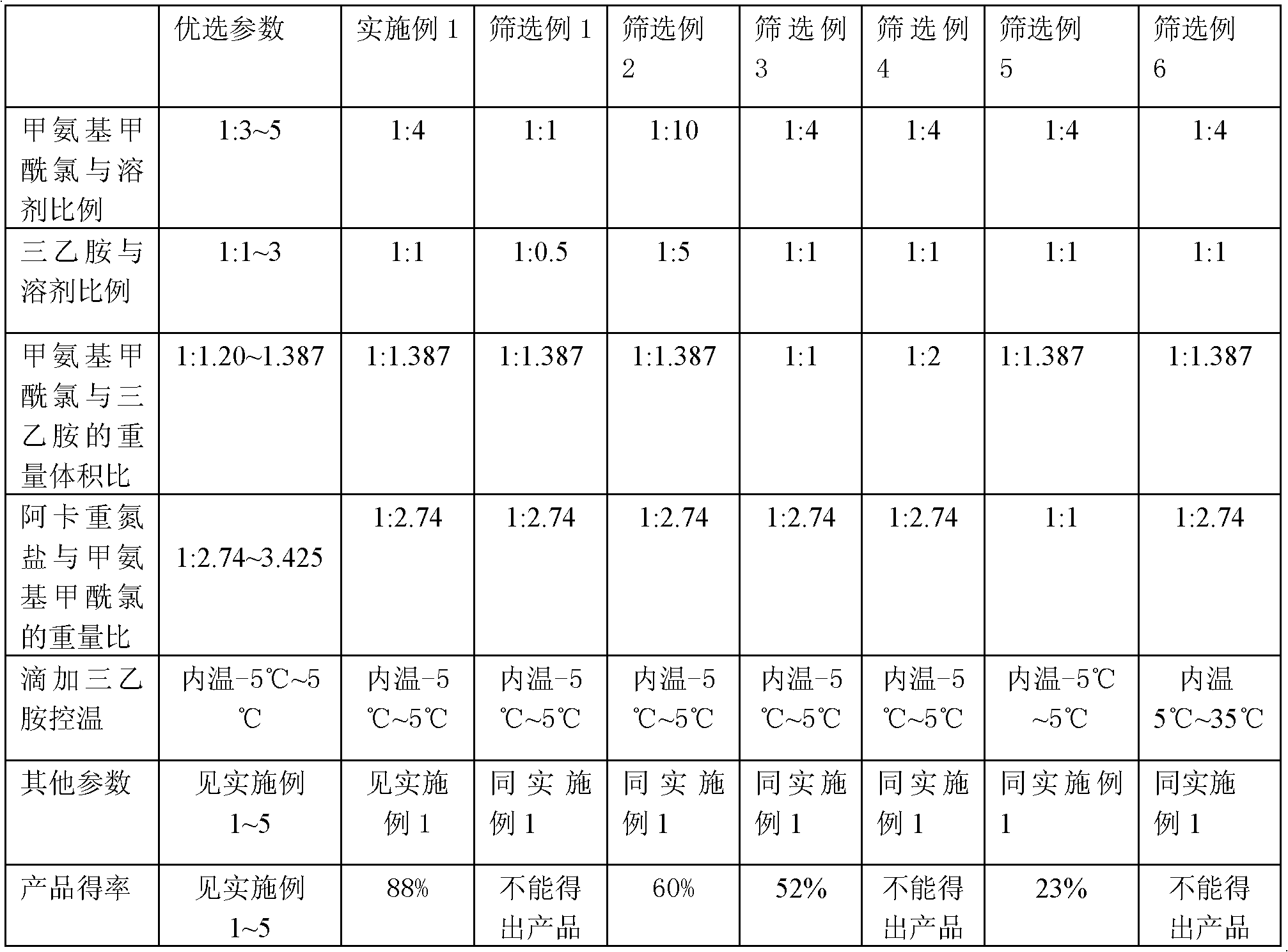
[0040] 1) Synthesis of crude temozolomide: Weigh 137g of carbamoyl chloride, add 274ml of DMF, stir to dissolve, under ice cooling, dropwise add triethylamine / DMF (190ml triethylamine / 190mlDMF) solution, dropwise, to obtain The reaction solution containing methyl isocyanate was directly added to the reaction solution with 50 g of Arca diazonium salt under stirring condition, protected from light, and stirred at room temperature for about 3 days. After suction filtration, the filtrate was concentrated to dryness and washed with absolute ethanol to obtain 62.8 g of crude temozolomide with a yield of 95%.
[0041] 2) Refining of crude temozolomide: Weigh 60 g of crude temozolomide, add 240 ml of DMSO, stir to dissolve under heating, add activated carbon for decolorization, and filter. After the filtrate was lowered to room temperature, 360 ml of acetone was added, and stirred for crystallization. Suction filtration, w...
Embodiment 2
[0047] Example 2 One-pot method for preparing Temozolomide
[0048] Synthesis of temozolomide crude product: Weigh 137g methylcarbamoyl chloride, add 411ml DMF, stir to dissolve, under ice bath cooling, add dropwise triethylamine / DMF (190ml triethylamine / 380mlDMF) solution, after the dropwise addition is complete, the isocyanate containing For the reaction solution of methyl cyanate, under the condition of stirring, directly add 50 g of aka diazonium salt into the reaction solution, and stir and react at room temperature in the dark for about 72 hours. After suction filtration, the filtrate was concentrated to dryness, washed with absolute ethanol, and dried to obtain 66.3 g of crude temozolomide, with a yield of 95%.
[0049] Purification of crude temozolomide: Weigh 60 g of crude temozolomide, add 180 ml DMSO, stir and dissolve under heating, add activated carbon for decolorization, and filter. The filtrate was stirred to cool down, 540ml of absolute ethanol was added, and ...
Embodiment 3
[0053] Example 3 One-pot method for preparing Temozolomide
[0054] The synthesis of temozolomide crude product: take by weighing 137g methyl carbamoyl chloride, add 411ml toluene, stir to dissolve, under ice-bath cooling, add dropwise triethylamine / toluene (190ml triethylamine / 380ml toluene) solution, dropwise, promptly obtains containing For the reaction solution of methyl isocyanate, under the condition of stirring, directly add 40 g of aka diazonium salt to the reaction solution, avoid light, and stir at room temperature for about 72 hours. After suction filtration, the filtrate was concentrated to dryness, washed with absolute ethanol, and dried to obtain 52.8 g of crude temozolomide, with a yield of 94%.
[0055] Purification of crude temozolomide: Weigh 30 g of crude temozolomide, add 90 ml of DMSO, stir and dissolve under heating, add activated carbon for decolorization, and filter. The filtrate was stirred to cool down, 180ml of acetone was added, stirred and crystal...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com