Glp-1-fc fusion protein formulation
A fusion protein and glp-1-fc technology, applied in peptide/protein components, drug combinations, medical preparations of non-active ingredients, etc., can solve problems such as unsuitable preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
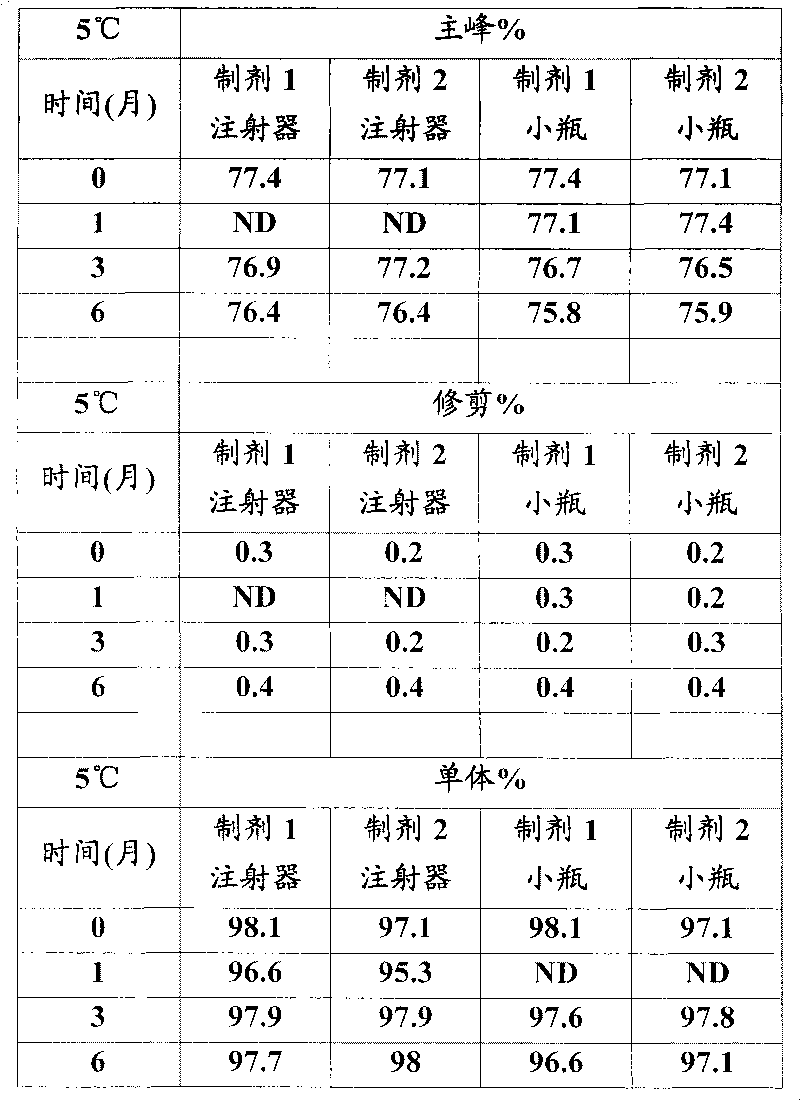
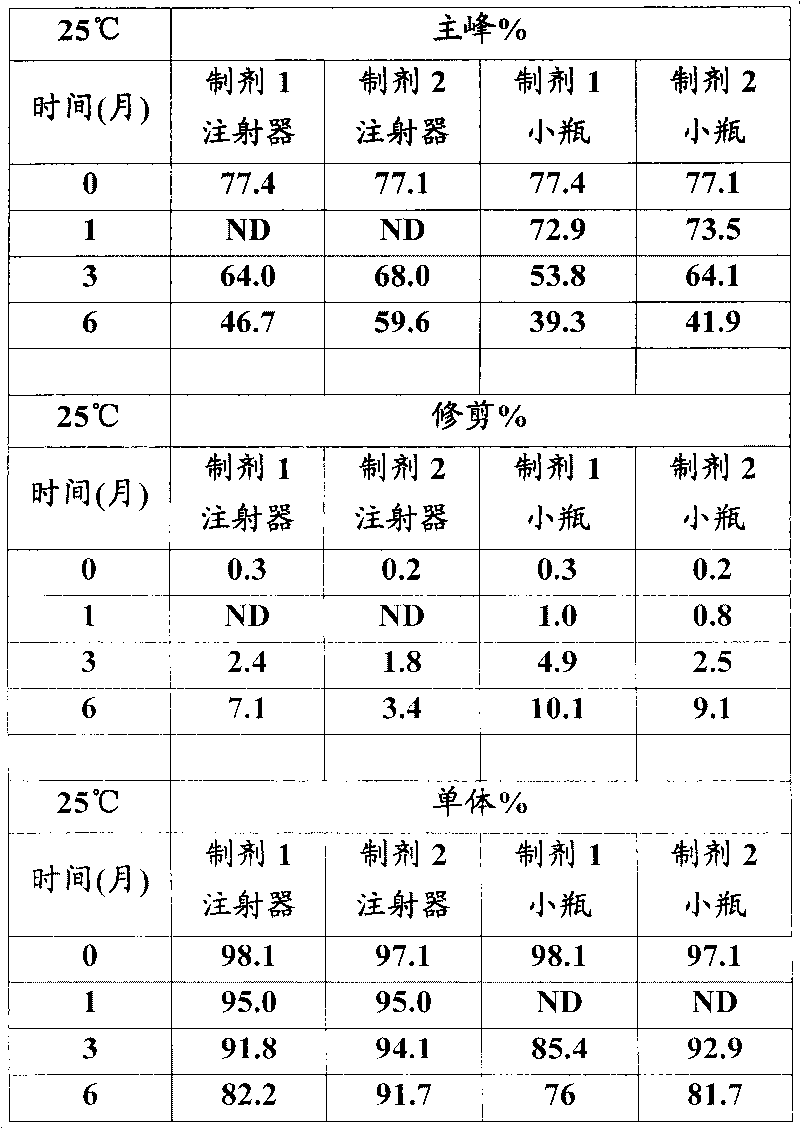
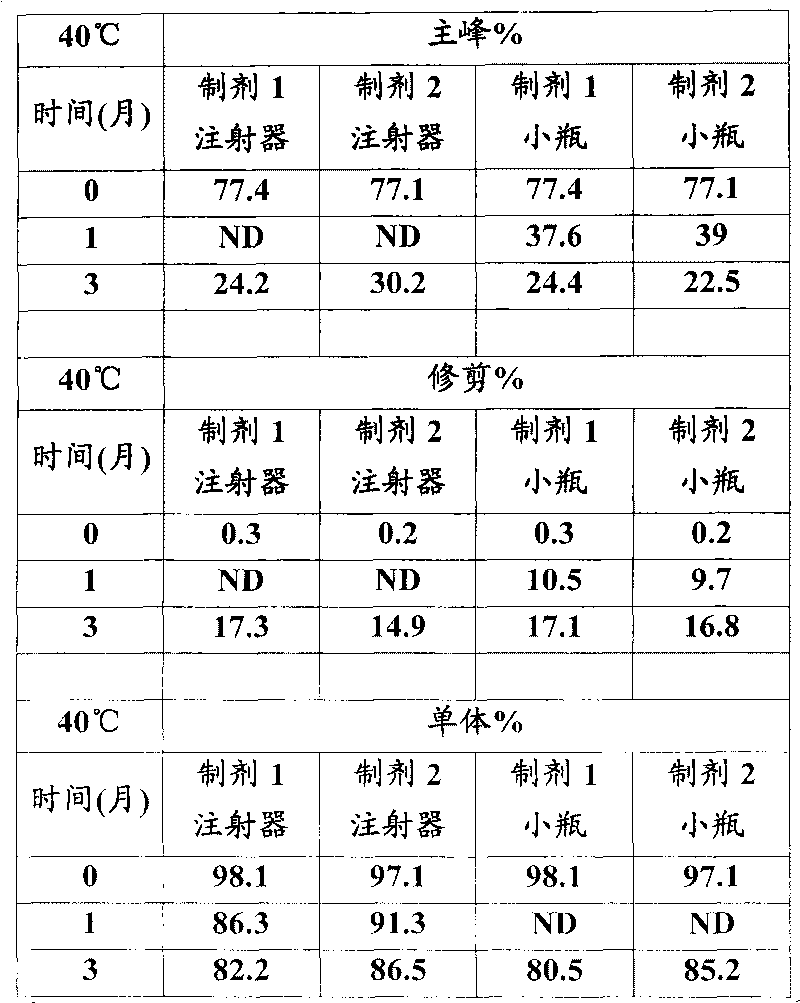
[0026] In vitro GLP-1 receptor activation assay
[0027] Using the CRE-luciferase system, HEK-293 cells stably expressing the human GLP-1 receptor were seeded in 96-well plates at 30,000 cells / well / 80 μL DMEM F12 medium with low serum. One day after seeding, 20 [mu]L aliquots of test proteins dissolved in 0.5% BSA were mixed with cells and incubated for 5 hours. For each test protein, 12 dilutions containing 3pM to 3nM were prepared typically at a 5X concentration and added to the cells to generate a dose response curve from which the EC was determined. 50 value. After incubation, 100 μL of luciferase reagent was added directly to each plate and mixed gently for 2 minutes. The plate was placed in a Tri-lux luminometer and the light output due to luciferase expression was calculated.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com