Phamaceutical/cosmetic compositions comprising hyaluronic acid and treatment of dermatological conditions therewith
a technology of hyaluronic acid and cosmetics, which is applied in the direction of drug compositions, biocide, dermatological disorders, etc., can solve the problems of wrinkles, decreased amount and quality, irregular distribution of melanin in the epidermis, etc., and achieves the effect of reducing the number, increasing the bioavailability of hyaluronic acid, and high efficacy in filling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
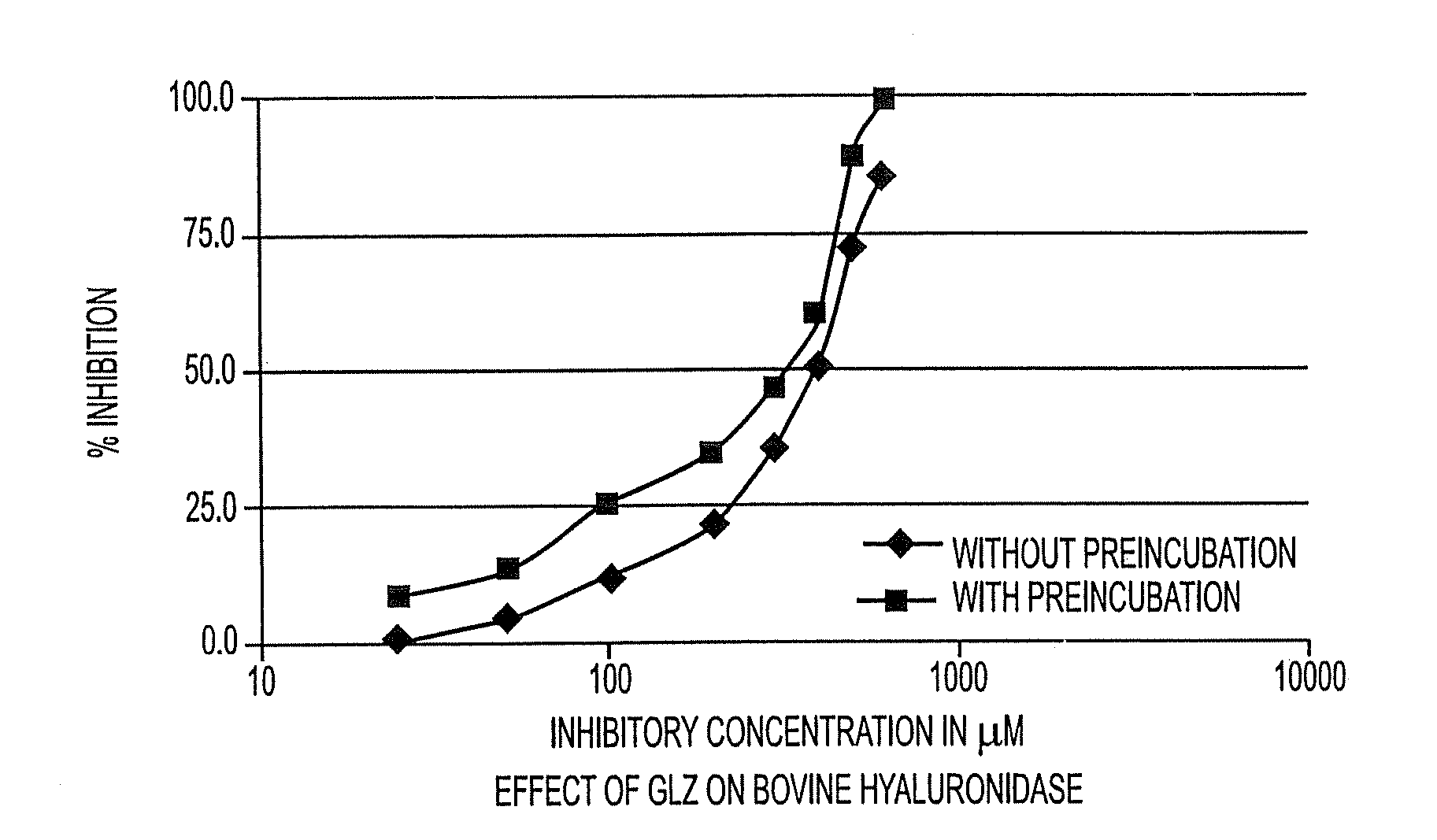
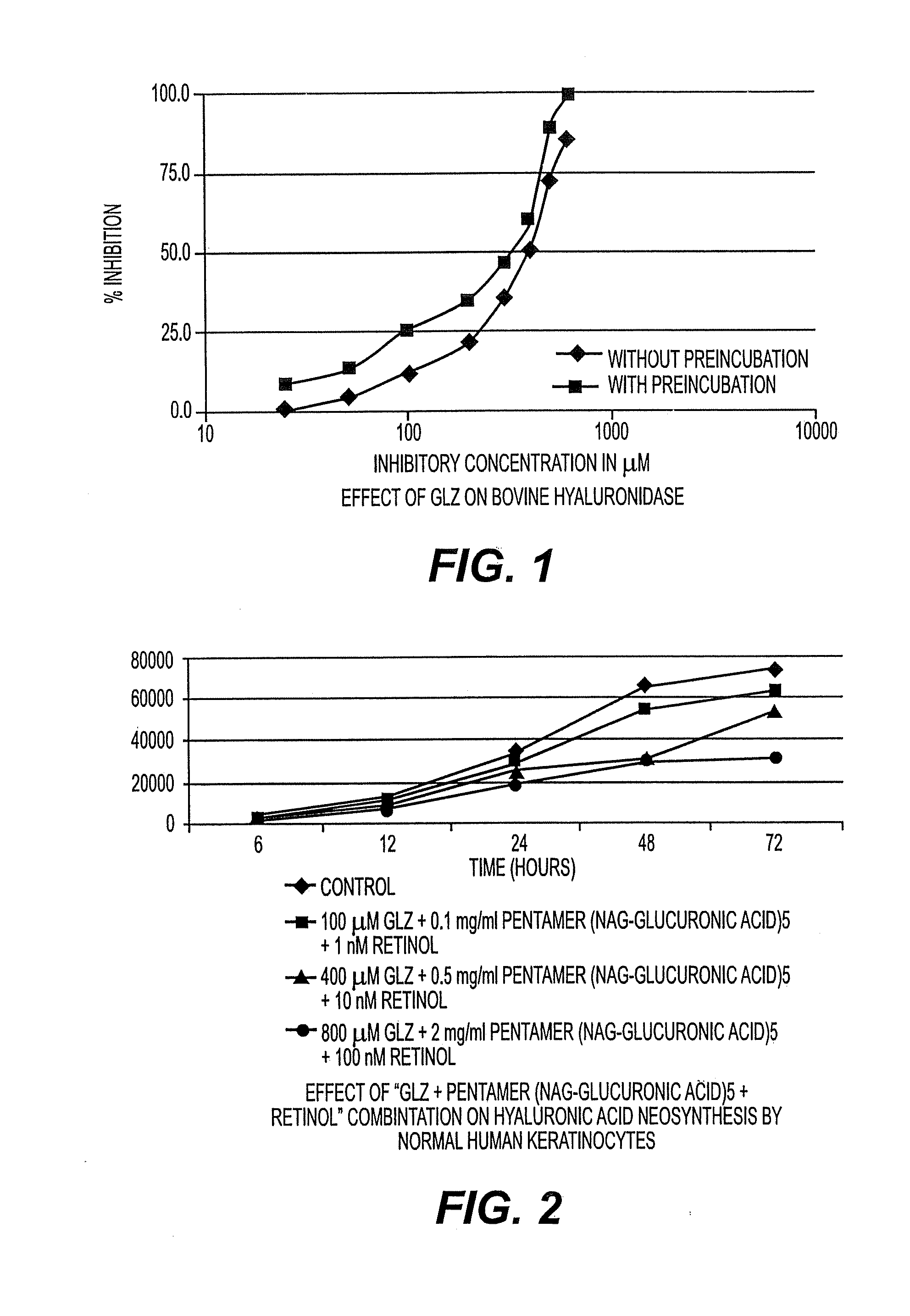
Inhibitory Effect of Glycyrrhizin (GLZ) on Hyaluronidase Activity of Bovine Origin
[0097]Determination of the IC50 of GLZ, with or without Pre-Incubation at 37° C.:
[0098]GLZ, at various concentrations, is or is not pre-incubated for 20 minutes at 37° C. in the presence of the enzyme. The enzyme reaction is triggered by adding the hyaluronic acid solution (time T0). After incubation for 20 minutes, the non-hydrolyzed hyaluronic acid is precipitated by adding acidic bovine albumin solution.
[0099]In order to verify that the pre-incubation step has no effect on the stability of the hyaluronidase, an aliquot of a solution of the enzyme is placed at 37° C. for 20 minutes. Another aliquot is conserved in an ice bath for 19 minutes, and is then incubated at 37° C. for 1 minute. A solution of hyaluronic acid is then added to each aliquot (T0). After incubation for 15, or 45 minutes, the non-hydrolyzed hyaluronic acid is precipitated by addition of acidic bovine albumin solution.
[0100]Measurem...
example 2
Inhibitory Effect of the “GLZ+Pentamer (NAG-Glucuronic Acid)5+Retinol” Combination on the Neosynthesis of Hyaluronic Acid by Normal Human Keratinocytes
[0105]According to the prior art, it is accepted that an equilibrium pre-exists from the neosynthesis and the degradation of hyaluronic acid. In other words, the neosynthesis of hyaluronic acid is a reflection of its degradation: measuring the variations in one therefore amounts to measuring the variations in the other. For reasons of technical simplicity, the change in neosynthesis of hyaluronic acid in the presence of the “GLZ+pentamer (NAG-glucuronic acid)5+retinol” combination is measured, relative to the corresponding control.
[0106]The adult human keratinocytes (NHK) are isolated from a fragment of human skin collected after an abdominoplasty operation (subject CAOL, 38 years old).
[0107]The NHK are cultured to confluence as a monolayer in 24-well plates and sub-cultured. The NHK are used following the third passage.
[0108]The cult...
example 3
Composition No. 1
[0134]Injectable solution No. 1 containing the 4 components:
[0135]This composition is prepared in a manner that is conventional for those skilled in the art:
Hyaluronic acid2%Glycyrrhizin0.02%Pentamer (NAC-glucuronic acid)50.002%Retinol0.00001%Waterqs 100%
PUM
| Property | Measurement | Unit |
|---|---|---|
| Composition | aaaaa | aaaaa |
| Acidity | aaaaa | aaaaa |
| Degradation properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com