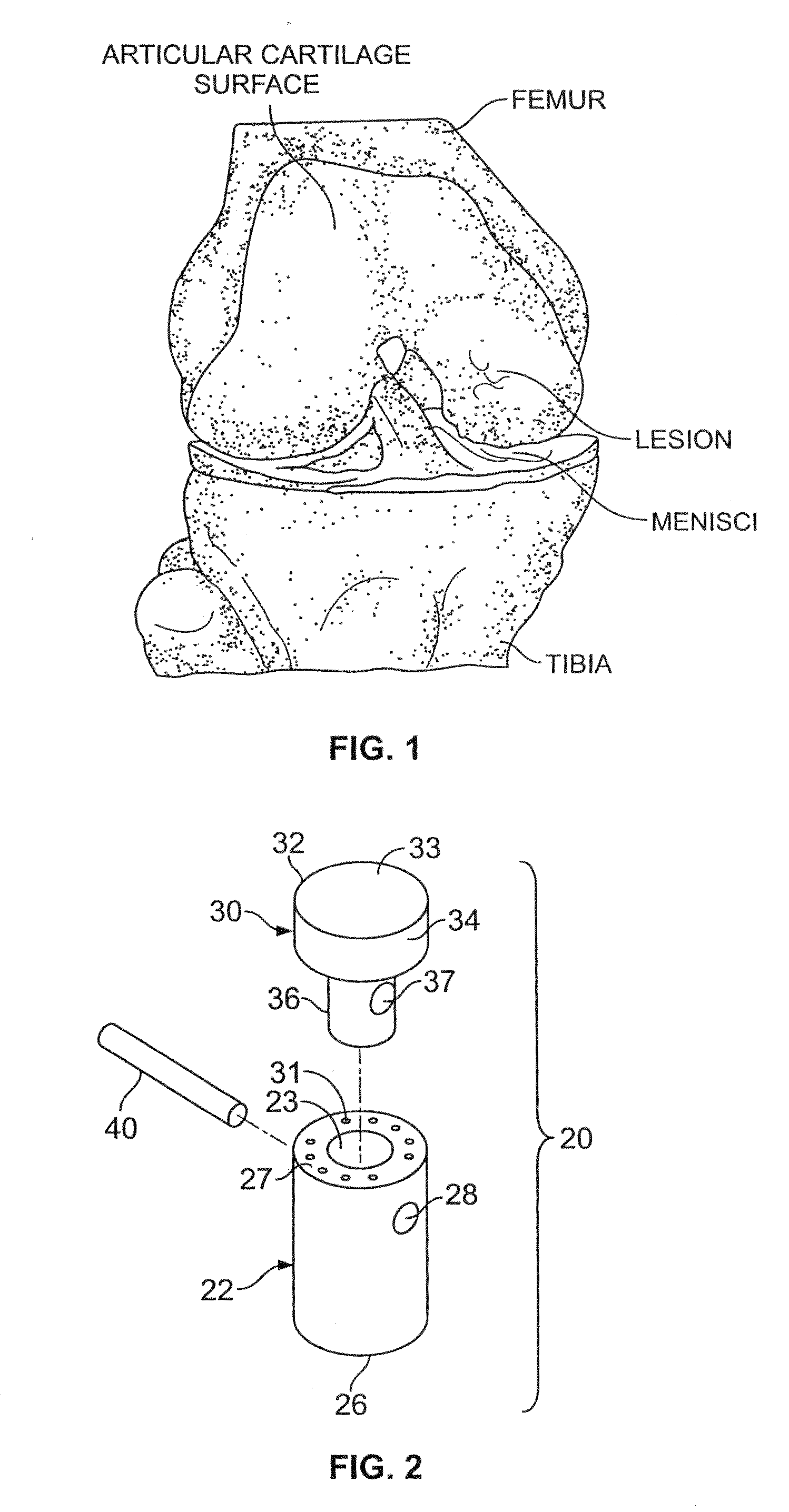
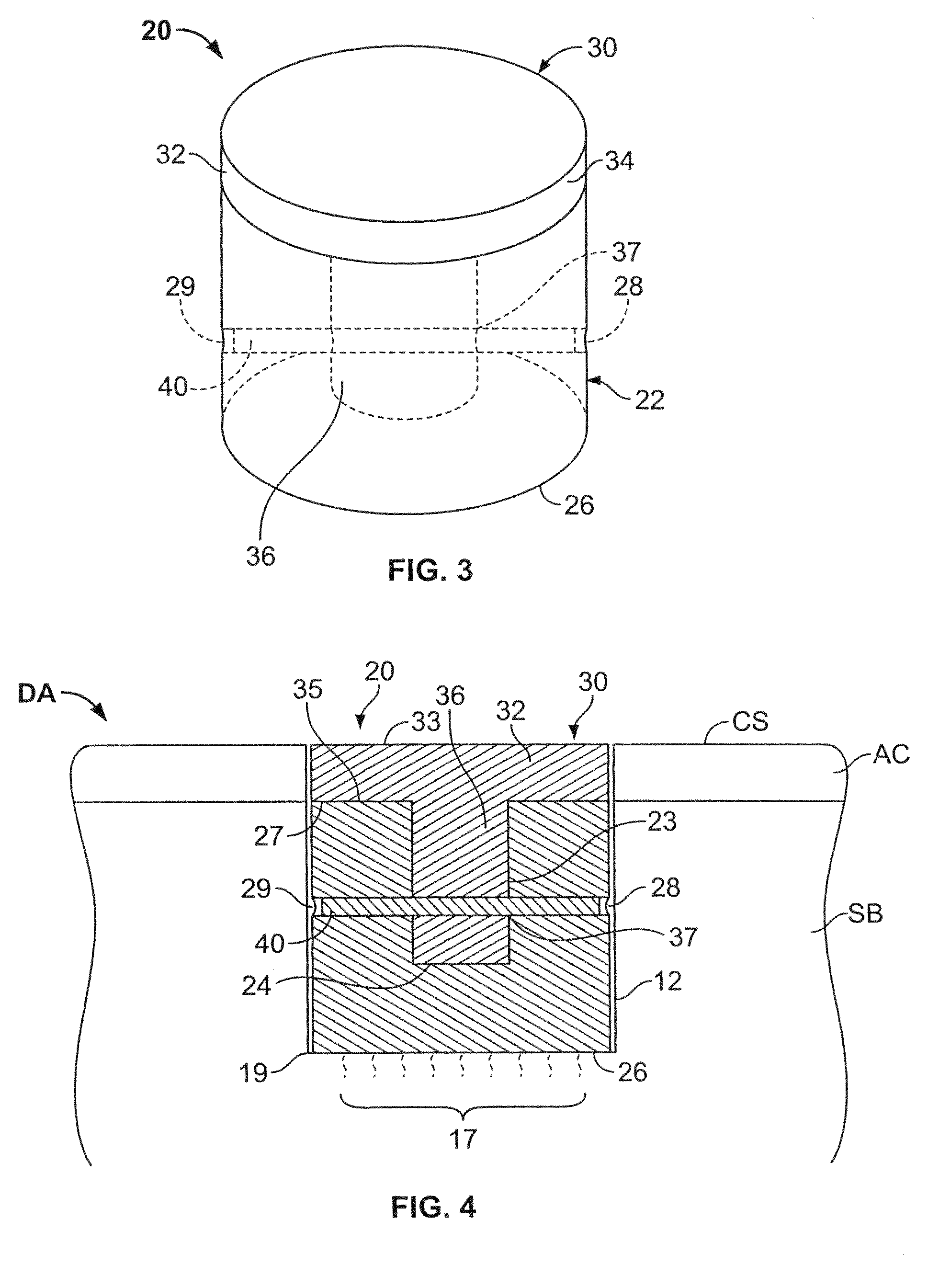
Cancellous constructs, cartilage particles and combinations of cancellous constructs and cartilage particles
a cancellous and cartilage technology, applied in the direction of prosthesis, drug compositions, peptide/protein ingredients, etc., can solve the problems of articular cartilage lesions generally not healing, pain or severe restriction of joint movement, and limited articular cartilage regeneration, so as to improve chondrogenesis and reduce fibrous tissue formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Measurement of Demineralized Construct Porosity
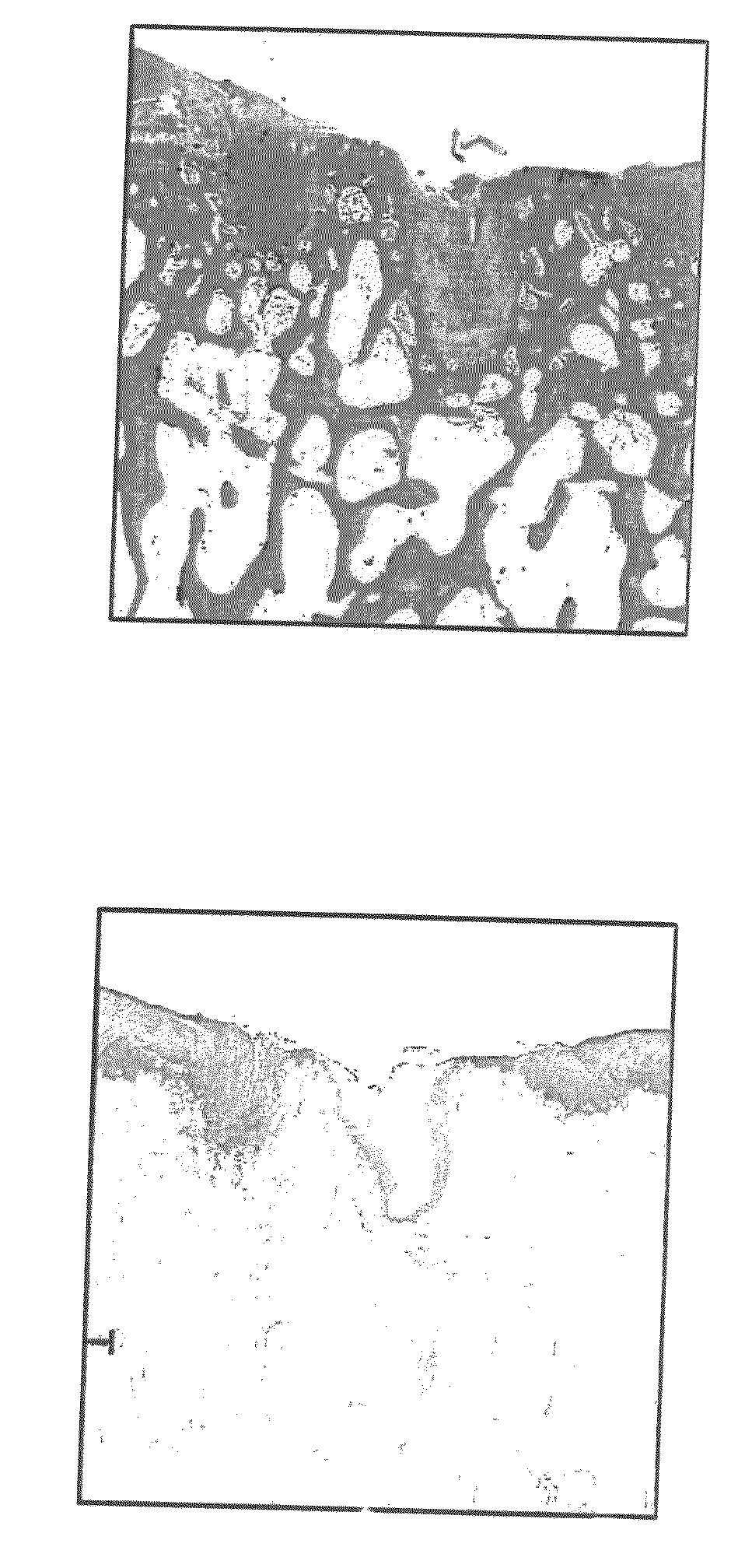
[0252]The percentage of porosity and average surface pore diameter of a cancellous construct demineralized cap member according to the present invention can be determined utilizing a microscope / infrared camera and associated computer analysis. The microscope / infrared camera was used to produce the images of FIGS. 34A and 34B, which provide a visual assessment of the porosity of the demineralized cap member of the constructs of the present invention. Such images were analyzed using suitable microscopy and image analysis software, for example, Image Pro Plus. The number and diameter of pores and the relative porosity of a demineralized cap member of the construct can be characterized using techniques known to those skilled in the art.
[0253]It is noted that the number and diameter of pores and the relative porosity of the demineralized cap members will vary from one tissue donor to another, and even within the tissue of one tissue donor, b...
example 2
Tissue Extraction and Particularization
[0254]A process of cartilage particle extraction may be applied to any of a number of different soft tissue types (for example, meniscus tissue). Cartilage is recovered from deceased human donors, and the tissue is treated with a soft tissue processing system for bioburden reduction, for example, as disclosed in U.S. Patent Application Publication No. US 2006 / 0275377 A1 of U.S. patent application Ser. No. 11 / 375,026, which is incorporated by reference herein in its entirety.
[0255]Fresh articular cartilage is removed from a donor using a scalpel, taking care to remove the cartilage so that the full thickness of the cartilage is intact (excluding any bone). Removed cartilage is then packaged in double Kapak® bags for storage until ready to conduct chemical cleaning of the allograft tissue, for example, in accordance with U.S. Patent Application Publication No. US 2006 / 0275377 A1. In one example, the cartilage can be stored in the refrigerator for...
example 3
Extraction of Proteins from Human Cartilage Using Extraction and Subsequent Dialysis
[0261]In another example, growth factors may be physically and / or chemically isolated from cartilage particles, and dialyzed using a suitable agent. The growth factors are thereby isolated for subsequent analysis and / or quantification. In one embodiment, 0.3 g of cartilage particles were weighed out for each donor. The cartilage particles were transferred to tubes containing 5 ml of extraction solution (4M Guanidine HCl in Tris HCI). The cartilage particles were incubated at 4° C. on an orbital shaker at 60 RPM for 24 hours, followed by dialysis (8 k MWCO membrane dialysis tube) in 0.05M TrisHCl or PBS for 15 hrs. at 4° C. The dialysis solution was then replaced and the dialysis continued for another 8 hrs. at 4° C. The post-dialysis extracts were stored at −70° C. until the ELISA was run.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| mean equivalent spherical diameter | aaaaa | aaaaa |
| aspect ratio | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com