Patents
Literature
47 results about "Osteogenic proteins" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
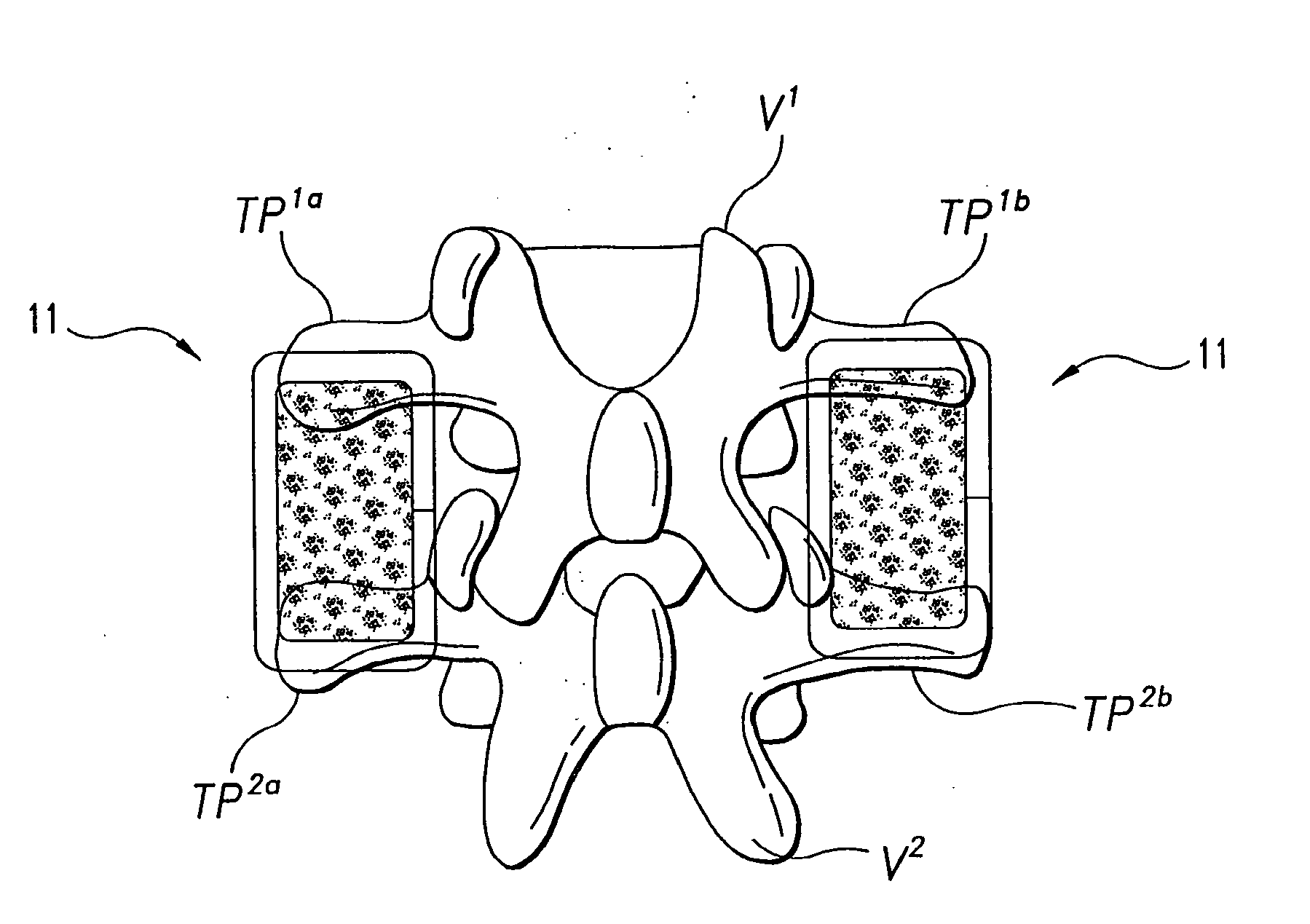
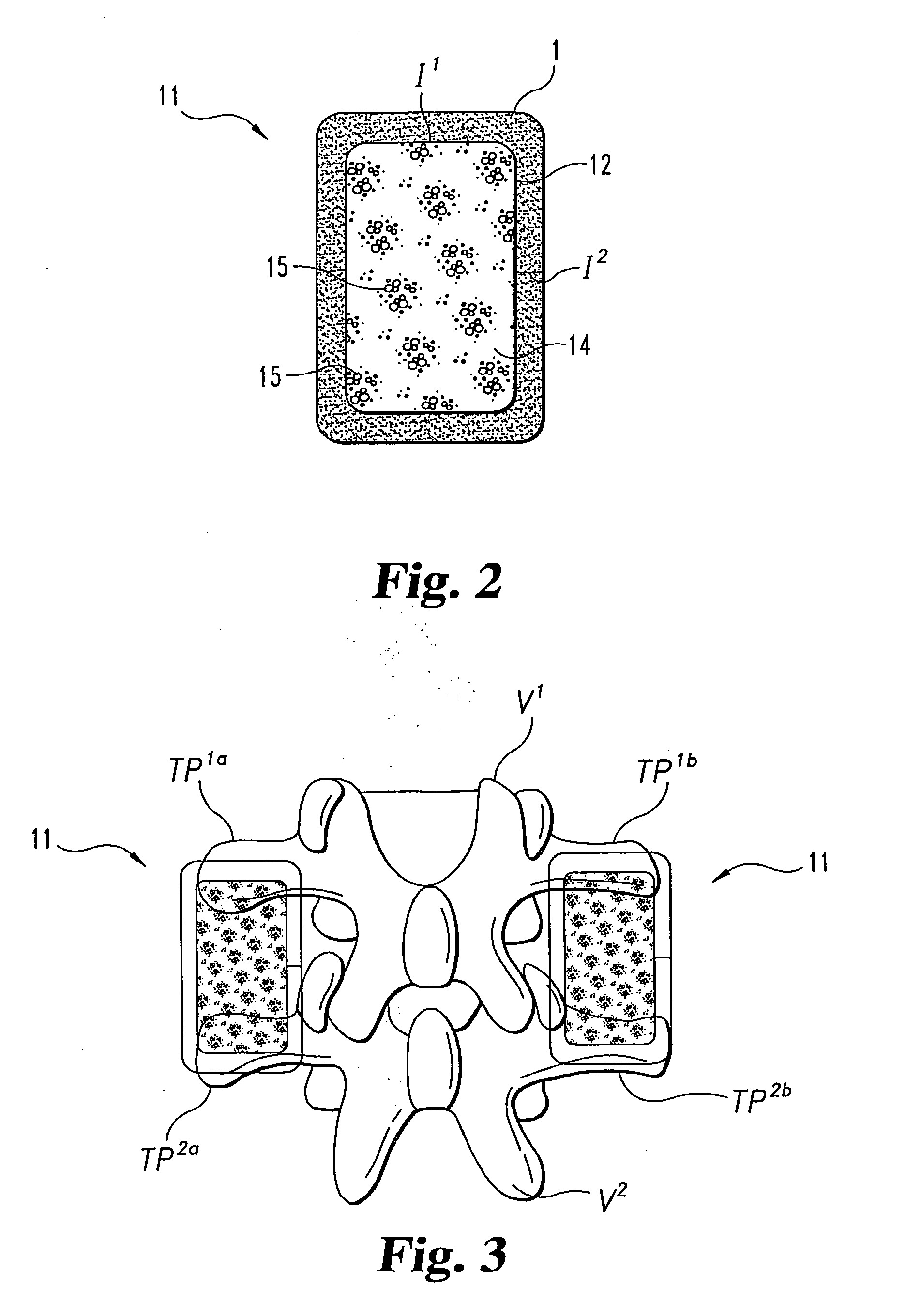
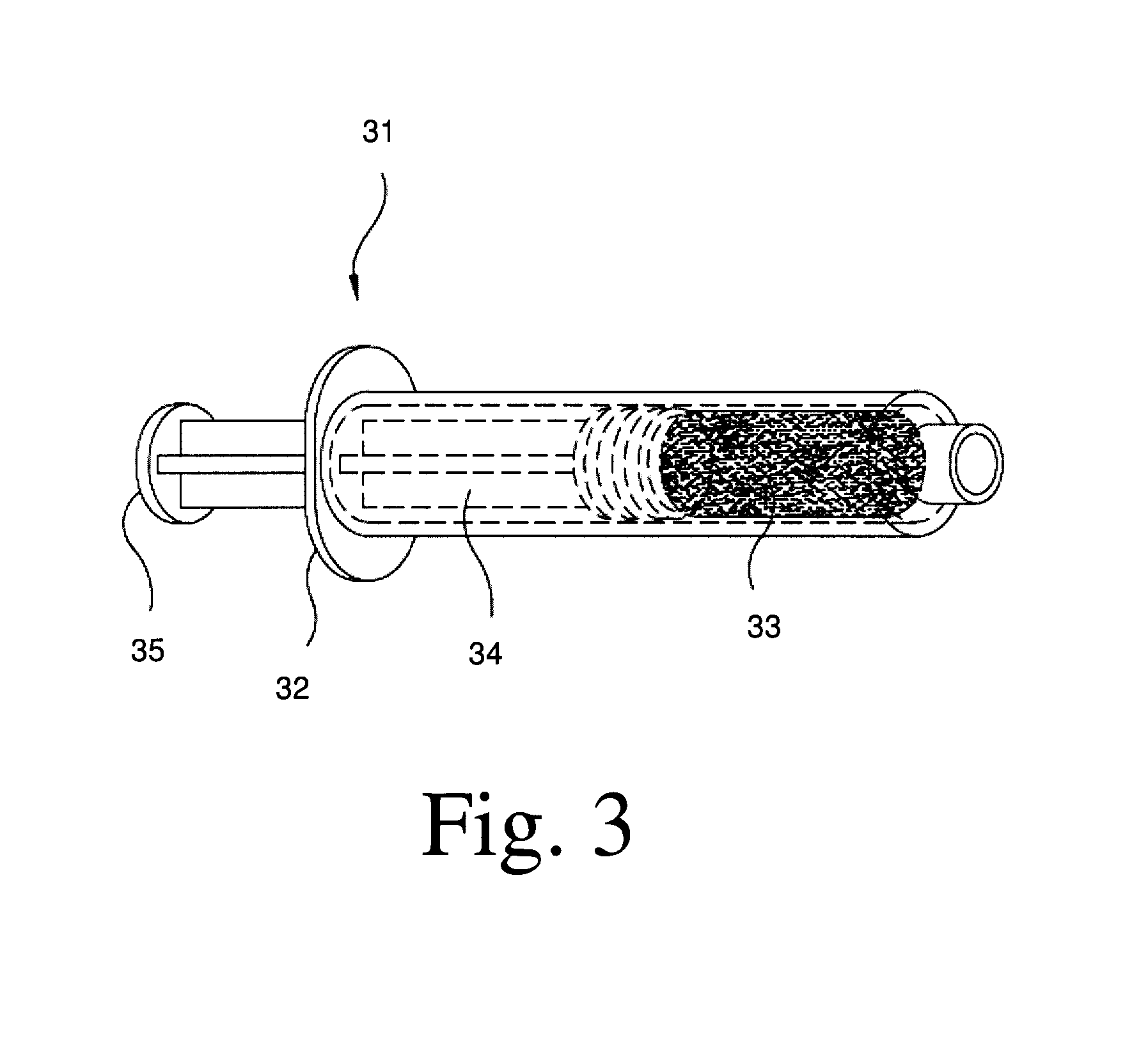
Osteogenic devices and methods of use thereof for repair of endochondral bone and osteochondral defects
InactiveUS7041641B2Avoid undesirable formationOvercome problemsPowder deliveryImpression capsRepair tissueNon union
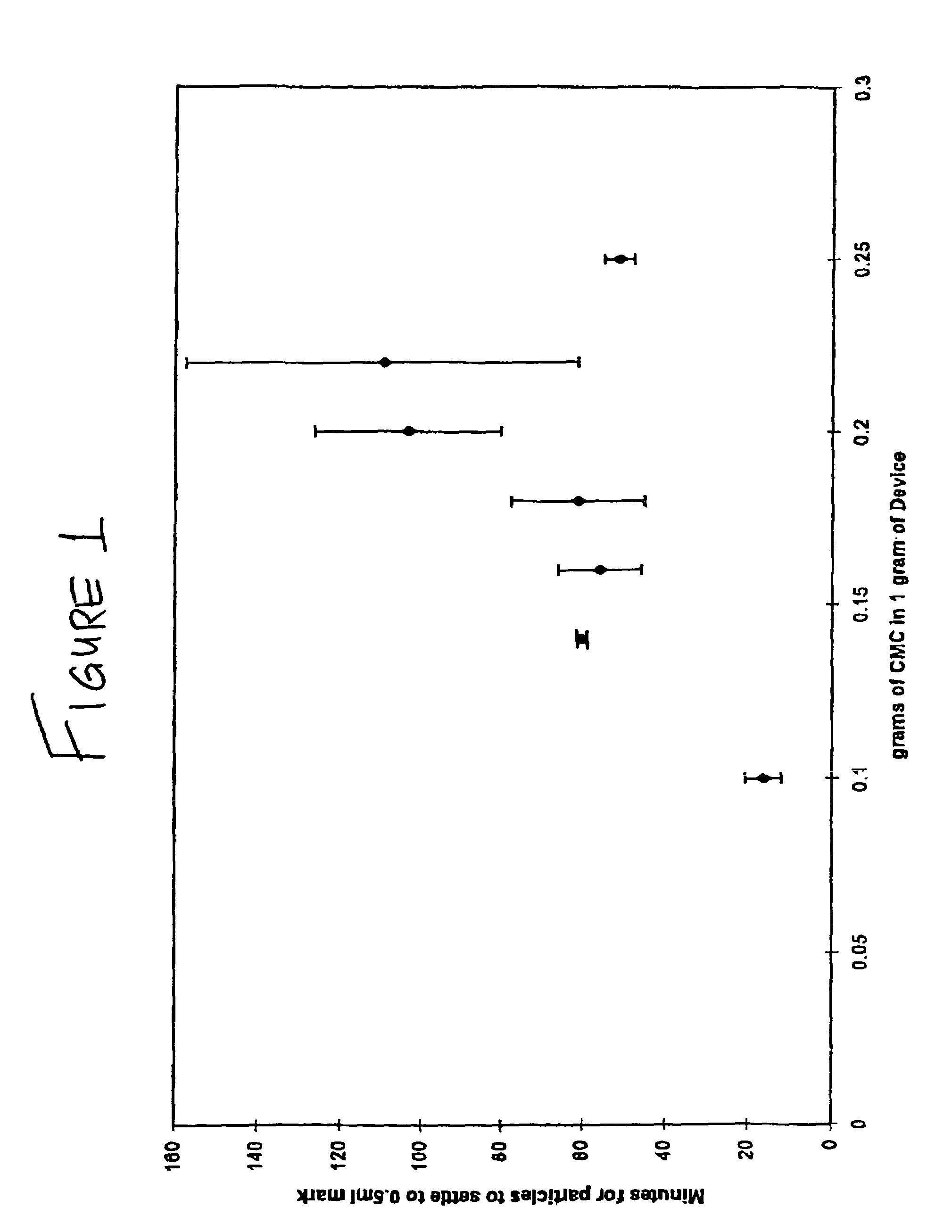
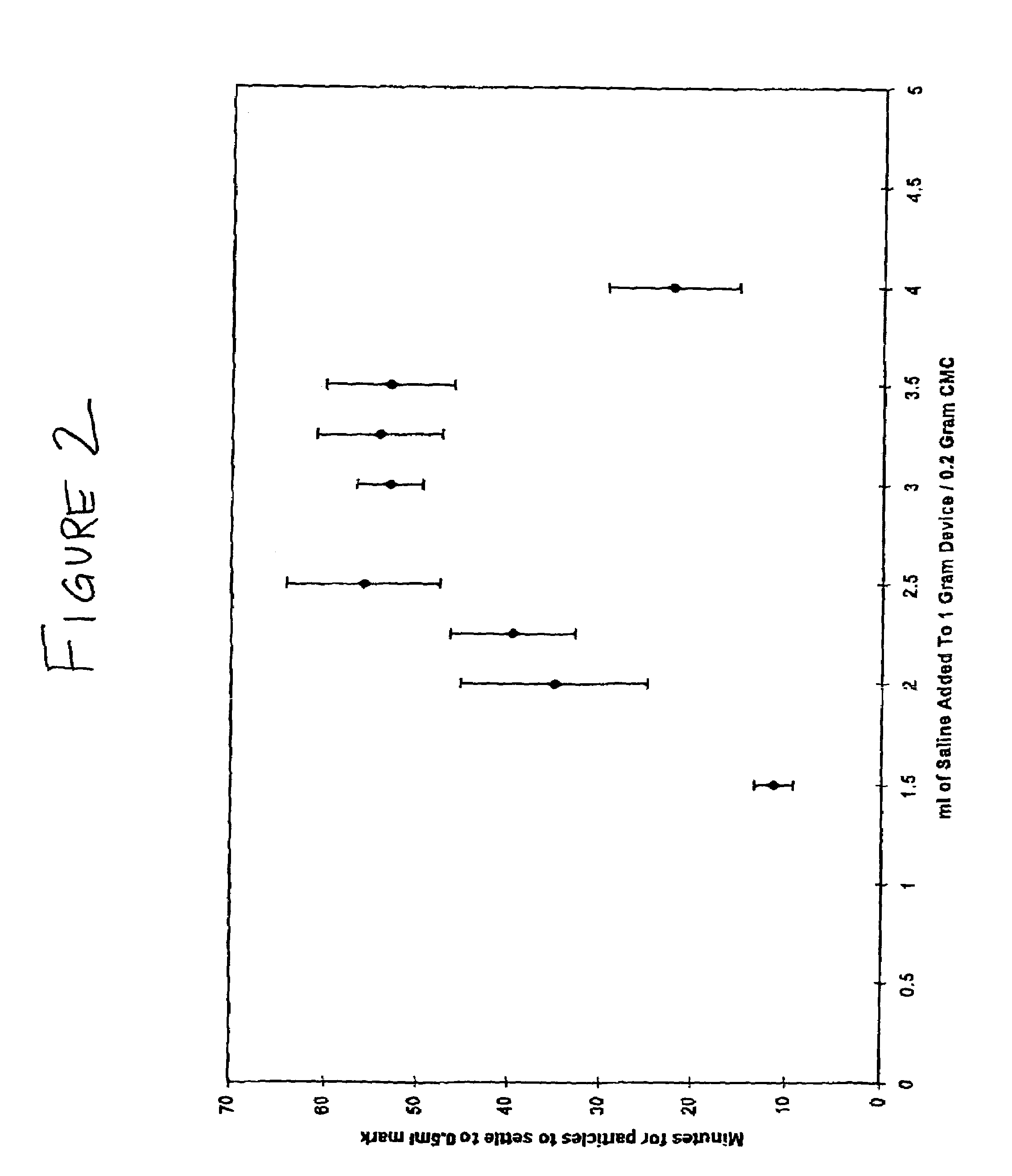
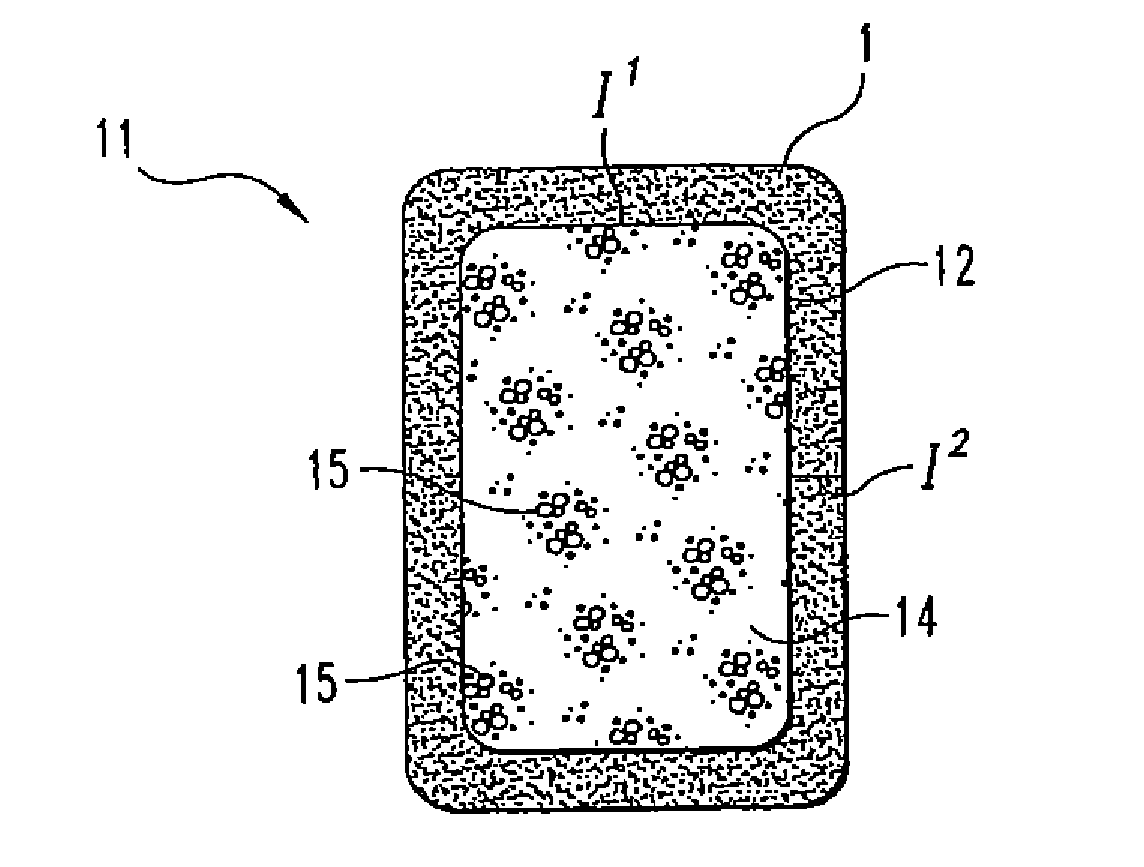
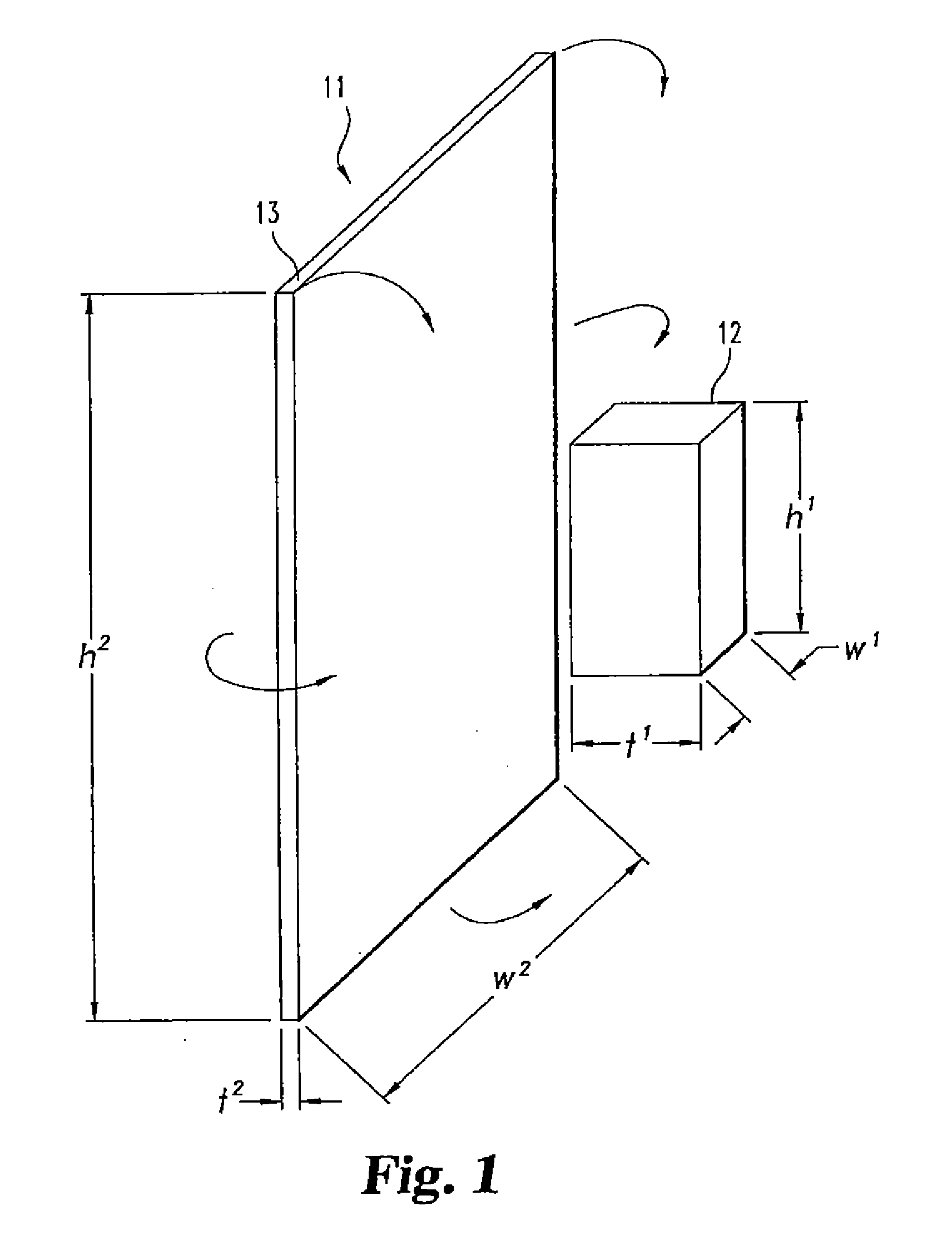
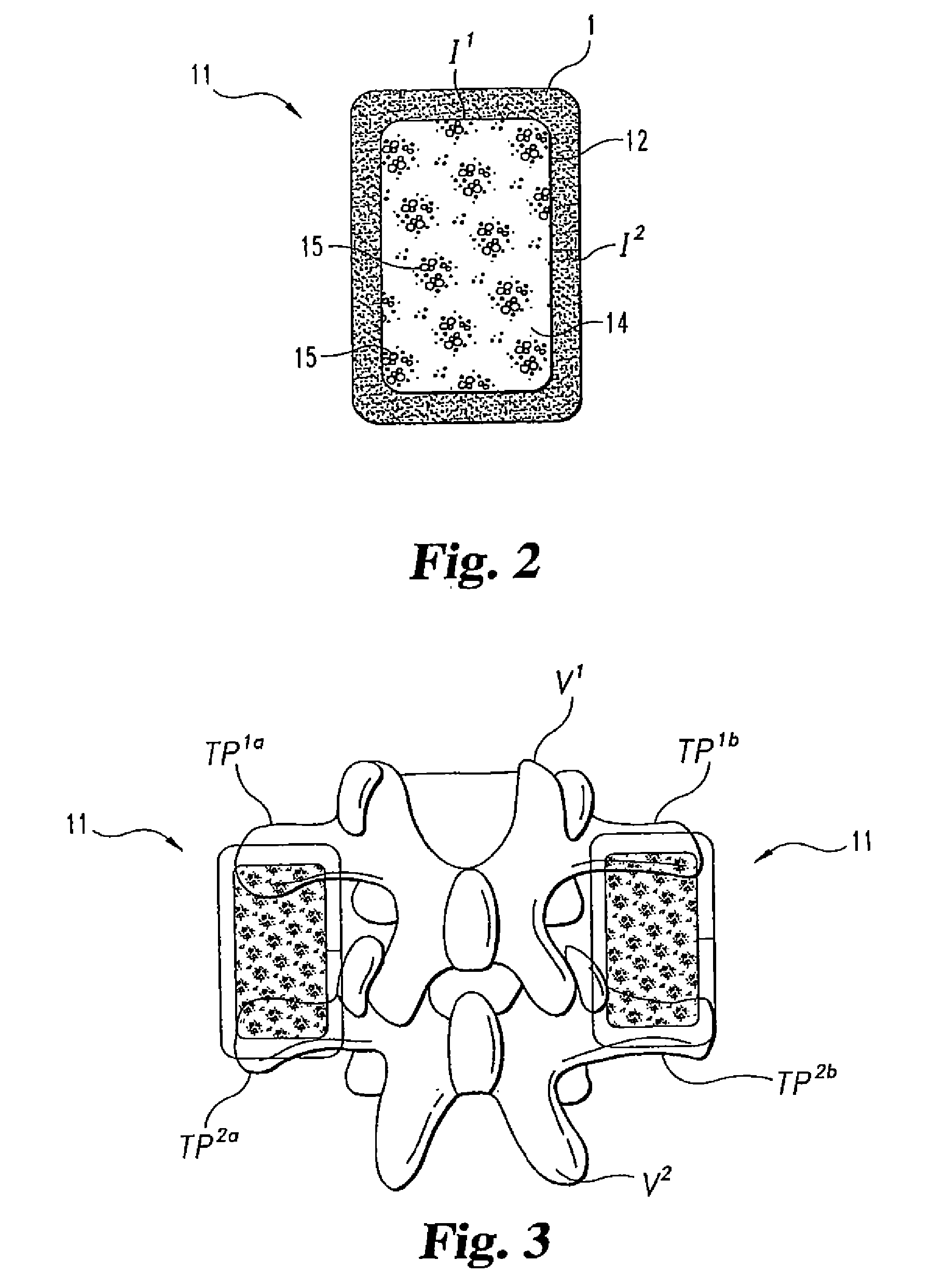
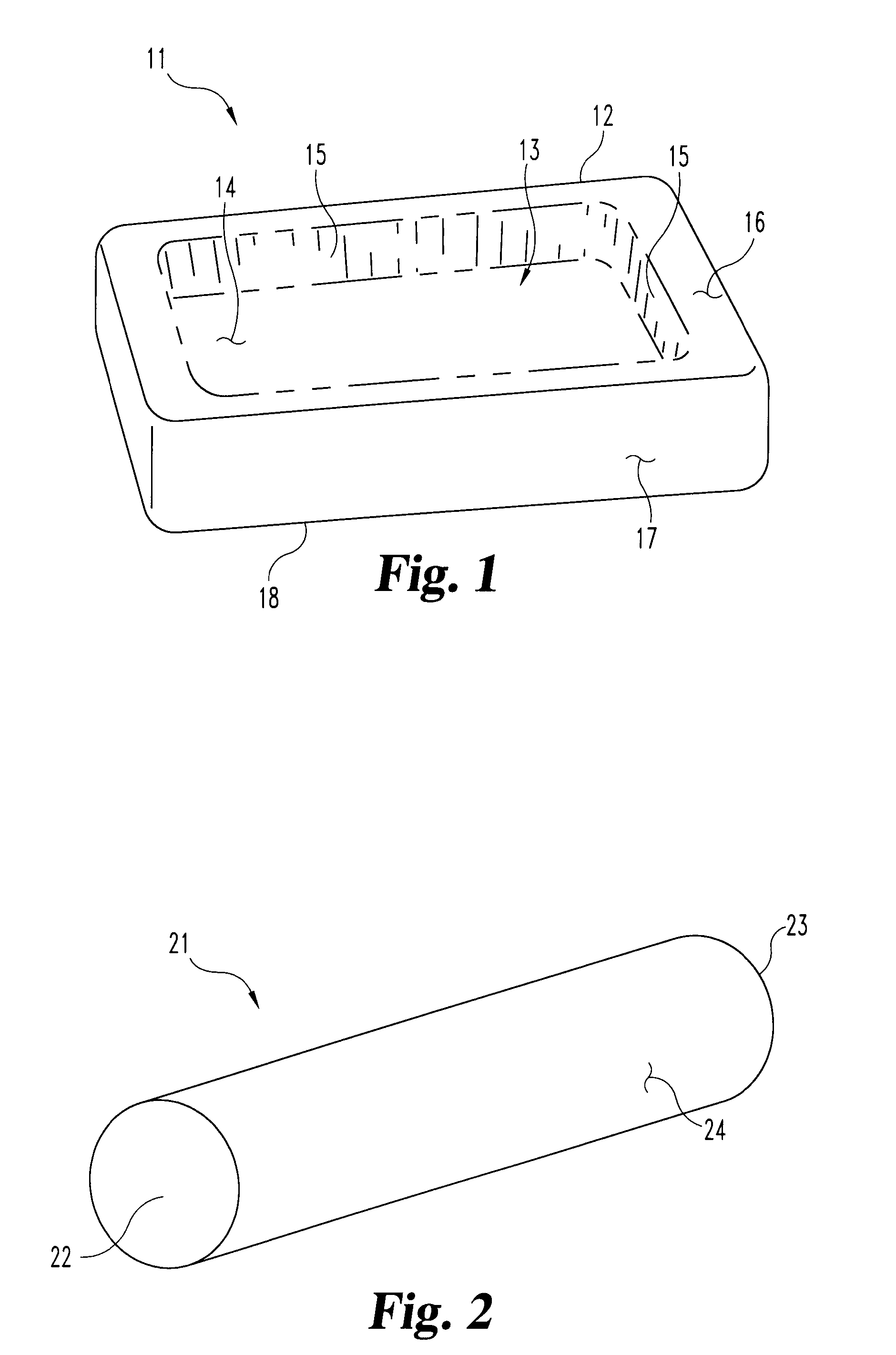
Disclosed herein are improved osteogenic devices and methods of use thereof for repair of bone and cartilage defects. The devices and methods promote accelerated formation of repair tissue with enhanced stability using less osteogenic protein than devices in the art. Defects susceptible to repair with the instant invention include, but are not limited to: critical size defects, non-critical size defects, non-union fractures, fractures, osteochondral defects, subchondral defects, and defects resulting from degenerative diseases such as osteochondritis dessicans.
Owner:MARIEL THERAPEUTICS
Malleable multi-component implants and materials therefor
ActiveUS20090246244A1High viscosityEfficiently formedPeptide/protein ingredientsSkeletal disorderFiberMedicine
Described are implantable, malleable medical materials comprising mineral particles, insoluble collagen fibers, and a gel-forming polysaccharide component and / or another added gel-former. The malleable medical materials can be used treat bone or other tissue defects in patients, including in conjunction with biologically active factors such as osteogenic proteins. Also described are methods and materials that can be used to prepare the malleable medical materials.
Owner:WARSAW ORTHOPEDIC INC
Osteogenic devices and methods of use thereof for repair of endochondral bone and osteochondral defects
InactiveUS20060177475A1Restore an osteochondral or a chondral defectInhibition formationImpression capsPeptide/protein ingredientsRepair tissueOsteogenic proteins
Disclosed herein are improved osteogenic devices and methods of use thereof for repair of bone and cartilage defects. The devices and methods promote accelerated formation of repair tissue with enhanced stability using less osteogenic protein than devices in the art. Defects susceptible to repair with the instant invention include, but are not limited to: critical size defects, non-critical size defects, non-union fractures, fractures, osteochondral defects, subchondral defects, and defects resulting from degenerative diseases such as osteochondritis dessicans.
Owner:MARIEL THERAPEUTICS
Repair of larynx, trachea, and other fibrocartilaginous tissues
InactiveUS6958149B2Severe inflammatory reactionSpeed up healing processImmobilised enzymesAnimal cellsOsteogenic proteinsIntervertebral disc
Provided herein are methods and devices for inducing the formation of functional replacement nonarticular cartilage tissues and ligament tissues. These methods and devices involve the use of osteogenic proteins, and are useful in repairing defects in the larynx, trachea, interarticular menisci, intervertebral discs, ear, nose, ribs and other fibrocartilaginous tissues in a mammal.
Owner:MARIEL THERAPEUTICS
Cohesive osteogenic putty and materials therefor
Described is an implantable medical material comprising a malleable, cohesive, shape-retaining putty including mineral particles, insoluble collagen fibers and soluble collagen. The medical material can be used in conjunction with biologically active factors such as osteogenic proteins to treat bone or other tissue defects in patients.
Owner:WARSAW ORTHOPEDIC INC
Method for repairing a defect in an intervertebral disc
InactiveUS7572440B2Severe inflammatory reactionSpeed up healing processBiocidePeptide/protein ingredientsOsteogenic proteinsNose
Provided herein are methods and devices for inducing the formation of functional replacement nonarticular cartilage tissues and ligament tissues. These methods and devices involve the use of osteogenic proteins, and are useful in repairing defects in the larynx, trachea, interarticular menisci, intervertebral discs, ear, nose, ribs and other fibrocartilaginous tissues in a mammal.
Owner:MARIEL THERAPEUTICS
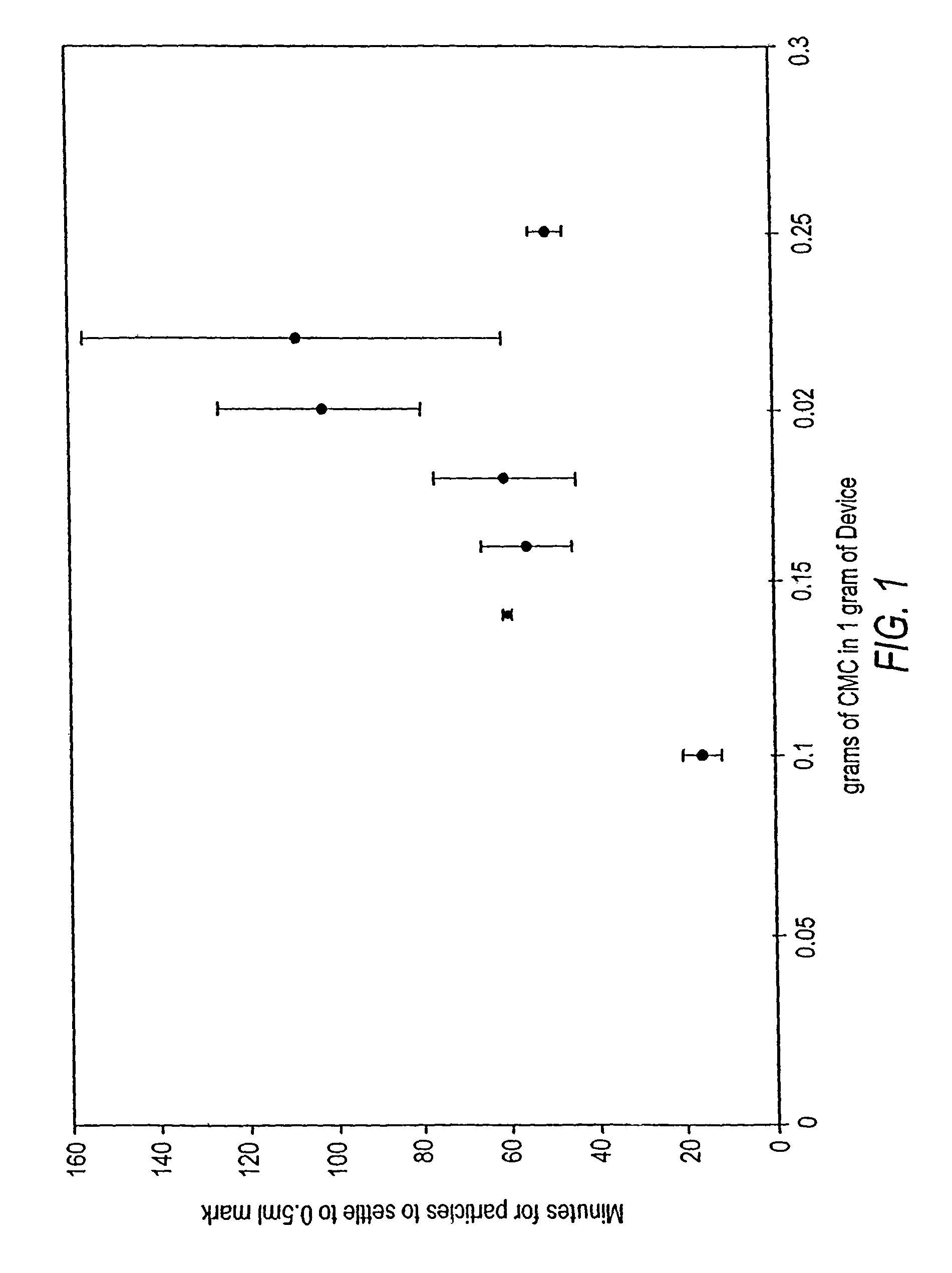
Calcium phosphate delivery vehicles for osteoinductive proteins
A composition for delivery of osteogenic proteins is disclosed. The composition comprises an osteogenic protein, a calcium phosphate material as a carrier, and an effective amount of an effervescent agent. Methods of making the compositions and methods of using the osteogenic compositions to treat osteoporotic and / or osteopenic bone are also disclosed.
Owner:ETEX +1
Rapid isolation of osteoinductive protein mixtures from mammalian bone tissue
InactiveUS7241874B2Reduce lossesImproved and simplifiedHydrolasesBone-inducing factorBones demineralizationWaste stream
A method for purifying bone-derived osteogenic proteins including a demineralization process, a protein extraction process, a high molecular weight ultrafiltration process, a low molecular weight ultrafiltration process, and a recovery process. The high and low ultrafiltration processes preferably select proteins having a nominal molecular weight between approximately 8 kilodaltons and approximately 50 kilodaltons. Processes of the present invention may be used to recover osteogenic proteins from bone demineralization waste streams.
Owner:ZIMMER ORTHOBIOLOGICS
Injectable calcium phosphate solid rods and pastes for delivery of osteogenic proteins
Osteogenic proteins are delivered via an injectable solid rod or hardenable paste. The formulation comprises a calcium phosphate material, an osteogenic protein, and optional additives and active ingredients such as a bone resorption inhibitor. Methods of making injectable pharmaceutical compositions and methods of using the osteogenic compositions to treat bone defects are also disclosed.
Owner:ETEX +1
Osteogenic Implants with Combined Implant Materials and Methods for Same
Described are osteogenic implants that include a first implant material covered at least in part by a second implant material carrying an osteogenic protein such as a bone morphogenic protein. The first implant material can comprise a mineral and provide an inner scaffolding portion for supporting bone ingrowth, and the second implant material can comprise a collagen or other sponge carrier covering the first implant material and having a liquid osteogenic protein formulation imbibed therein. Related implant materials and methods of preparation and use constitute additional aspects of the invention.
Owner:WARSAW ORTHOPEDIC INC
Repair of larynx, trachea, and other fibrocartilaginous tissues
InactiveUS20060029591A1Severe inflammatory reactionSpeed up healing processPowder deliveryPeptide/protein ingredientsOsteogenic proteinsNose
Provided herein are methods and devices for inducing the formation of functional replacement nonarticular cartilage tissues and ligament tissues. These methods and devices involve the use of osteogenic proteins, and are useful in repairing defects in the larynx, trachea, interarticular menisci, intervertebral discs, ear, nose, ribs and other fibrocartilaginous tissues in a mammal.
Owner:MARIEL THERAPEUTICS
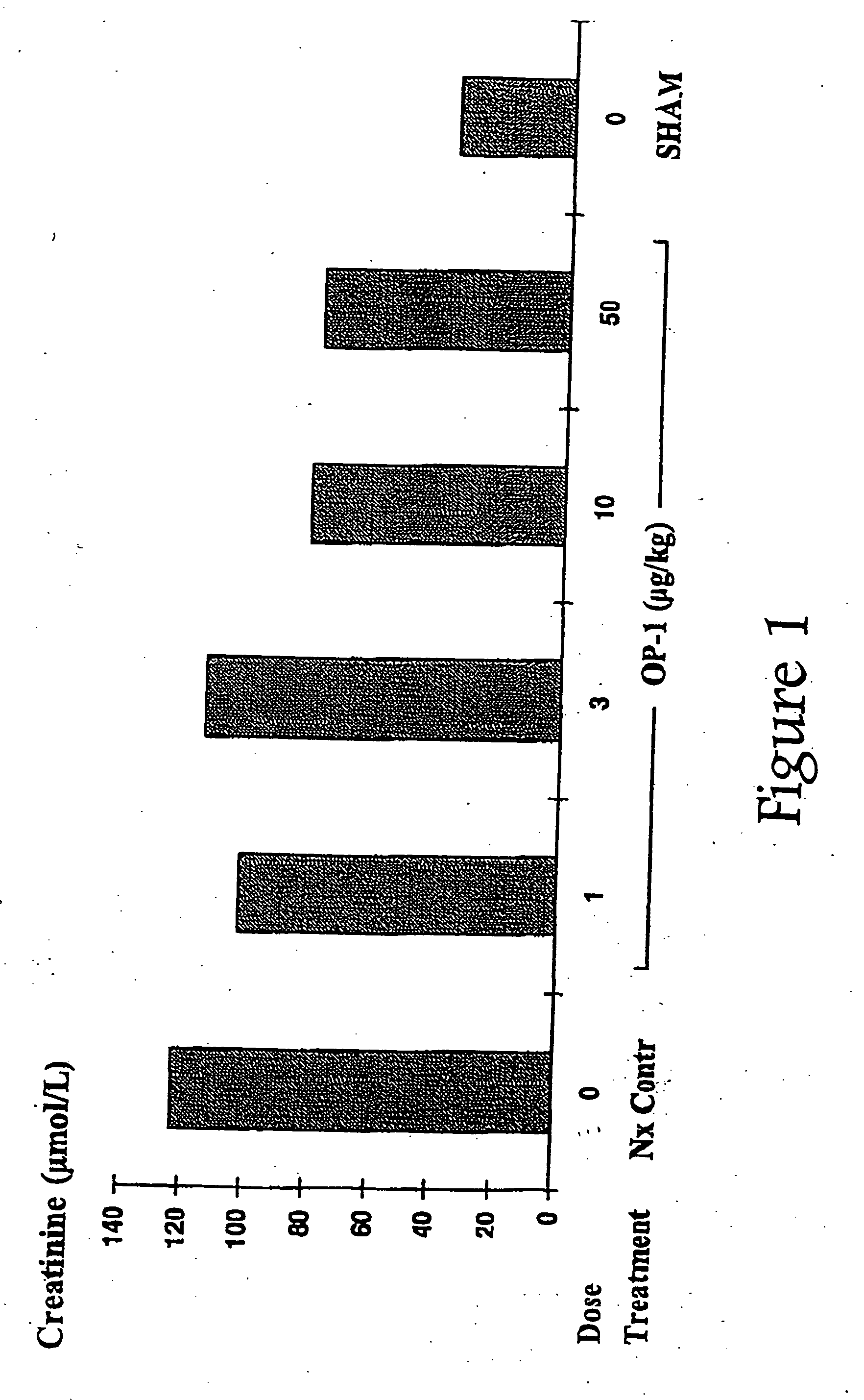
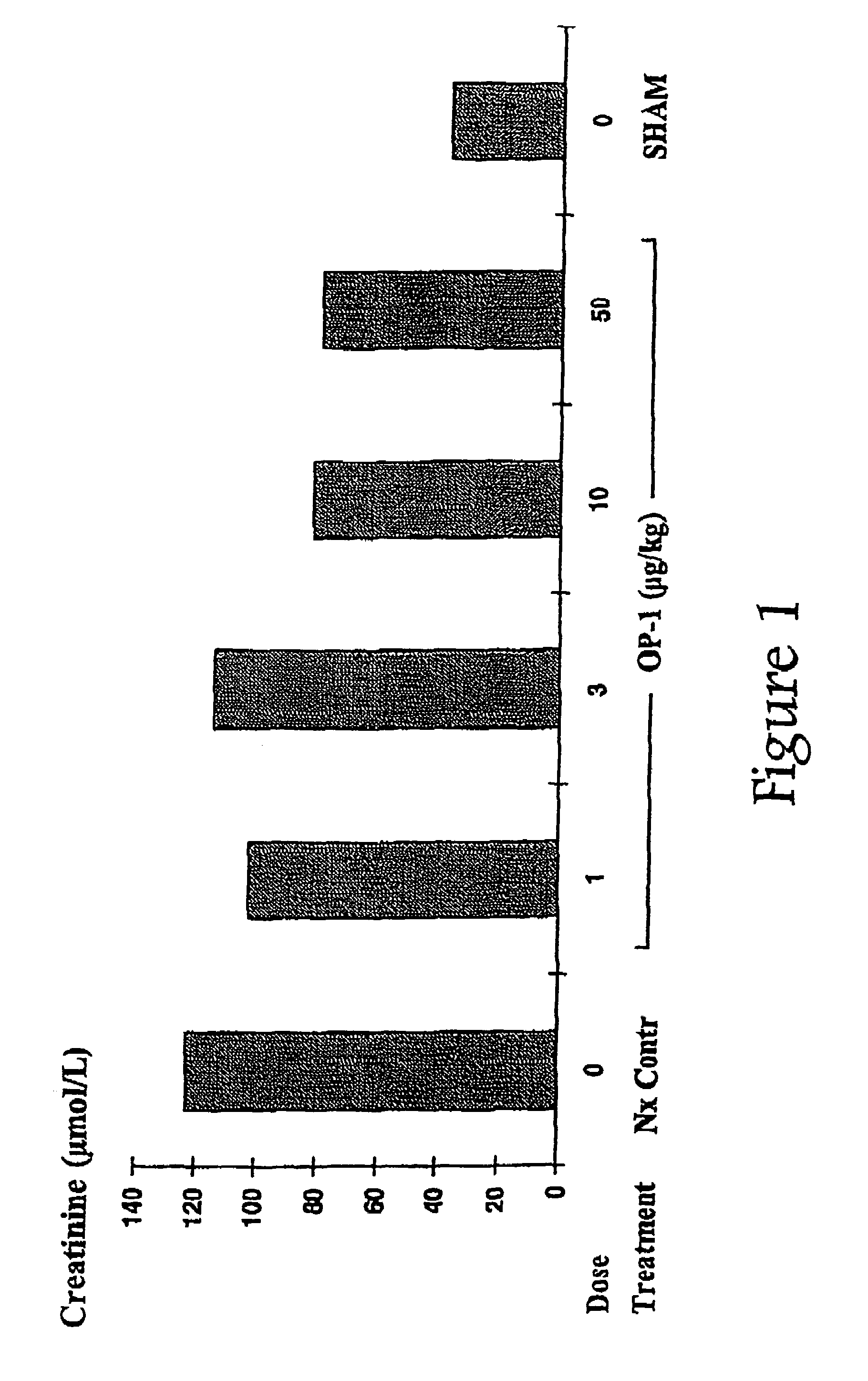
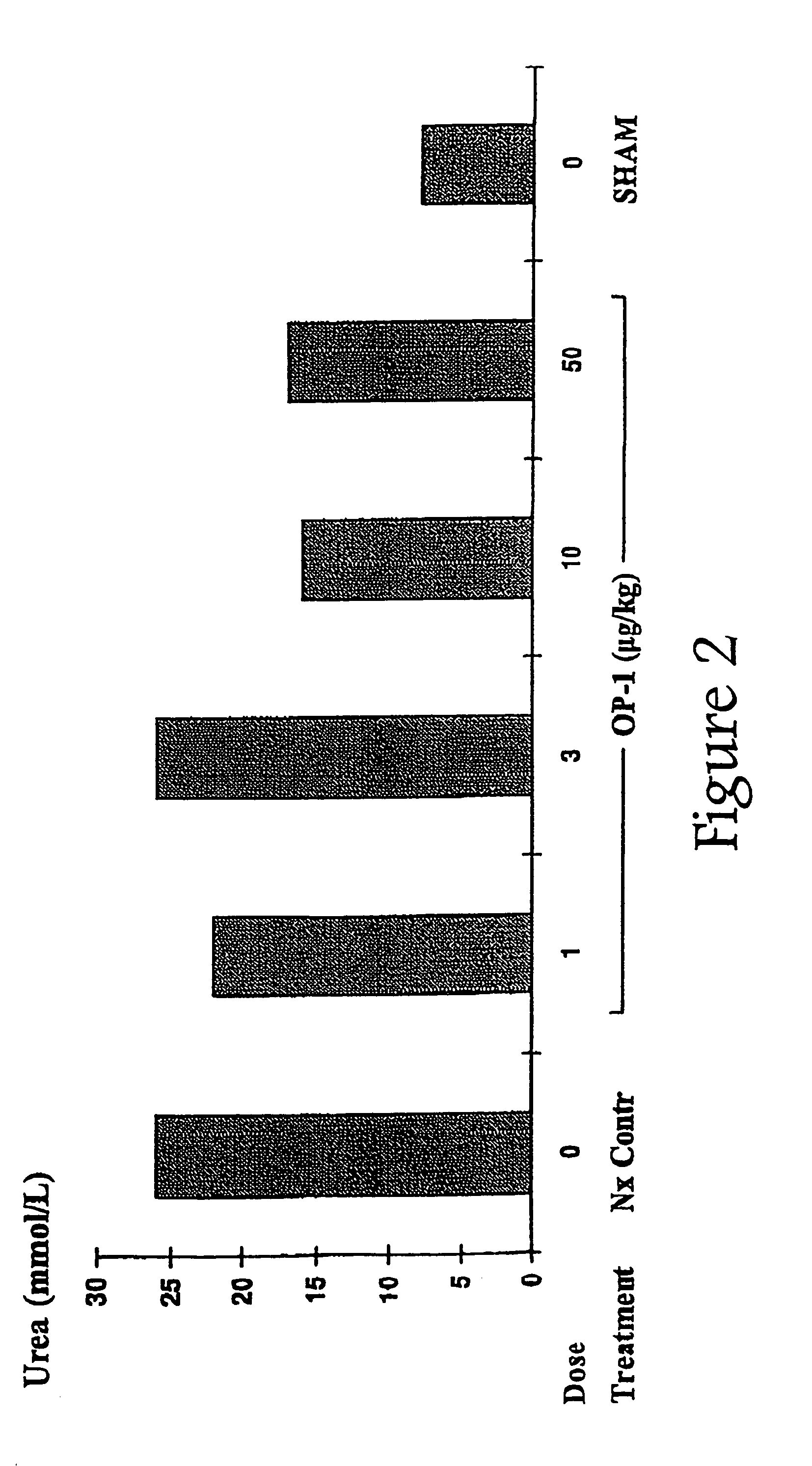
Novel therapies for chronic renal failure
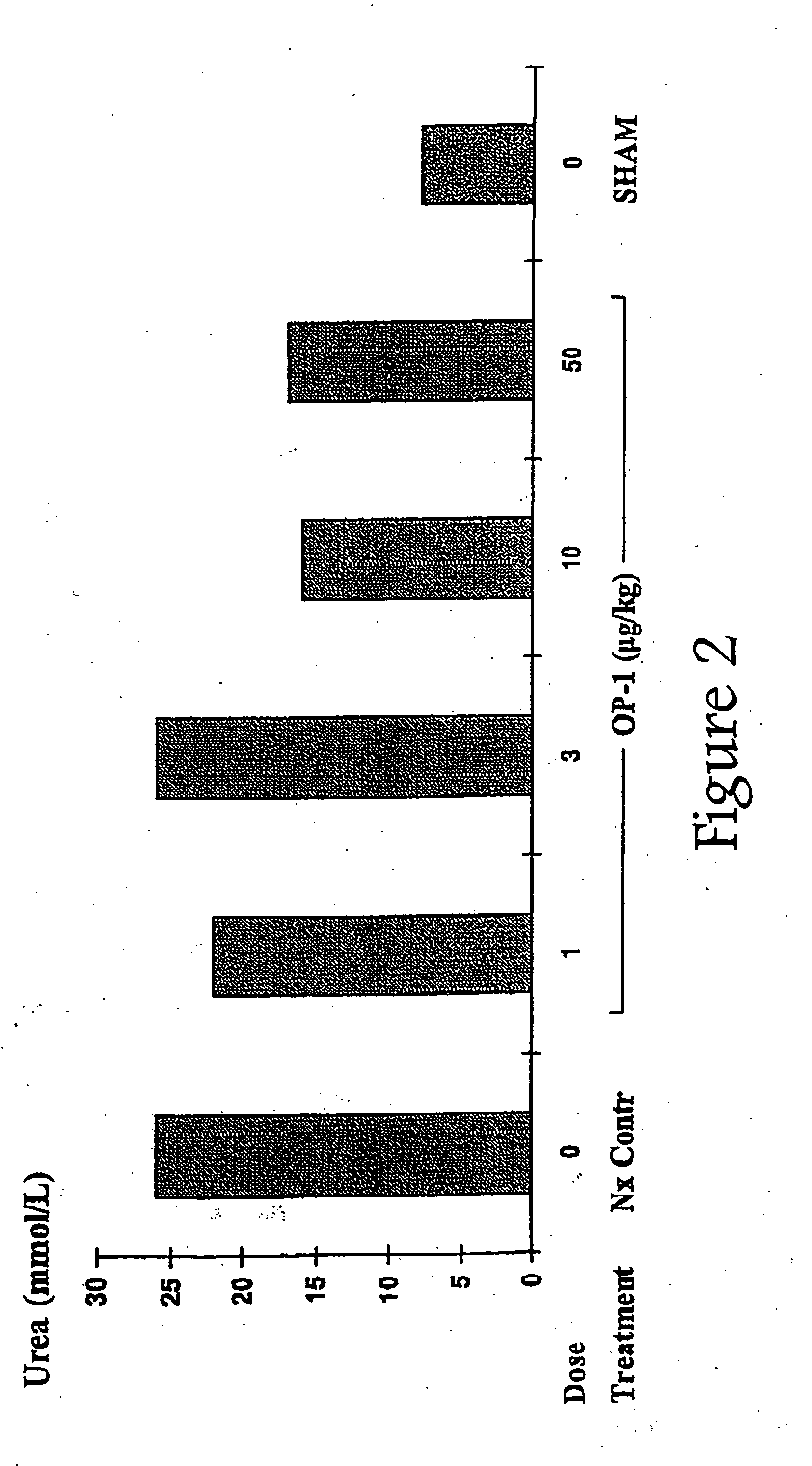
InactiveUS20050143304A1Preventing delaying needReducing necessary frequencyOrganic active ingredientsPeptide/protein ingredientsOsteogenic proteinsMorphogenesis
The present invention provides methods for the treatment, and pharmaceuticals for use in the treatment, of mammalian subjects in, or at risk of chronic renal failure, or at risk of a need for renal replacement therapy. The methods involve the administration of certain proteins of, or based upon, the osteogenic protein / bone morphogenetic protein (OP / BMP) family within the TGF-β superfamily of proteins.
Owner:MARIEL THERAPEUTICS
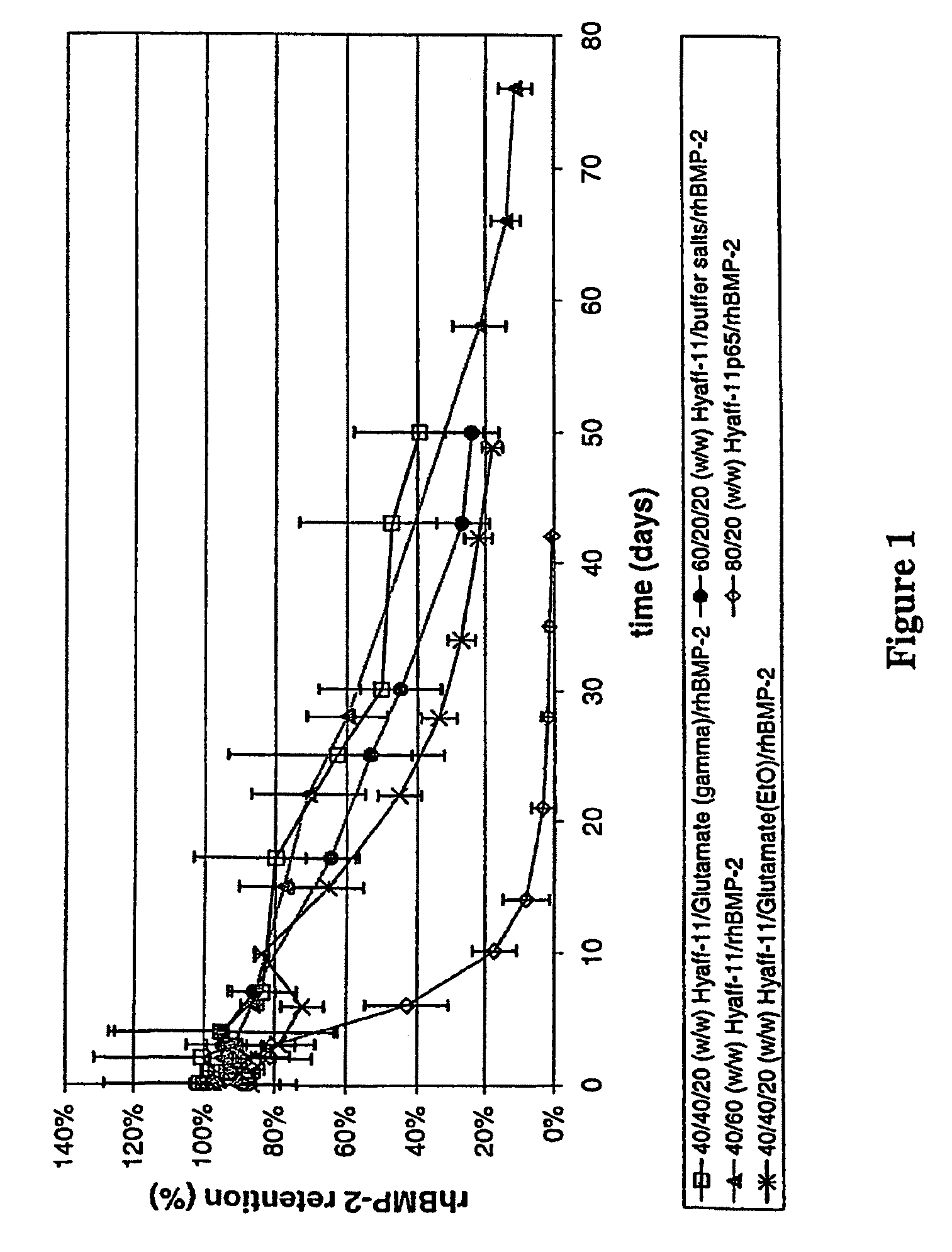
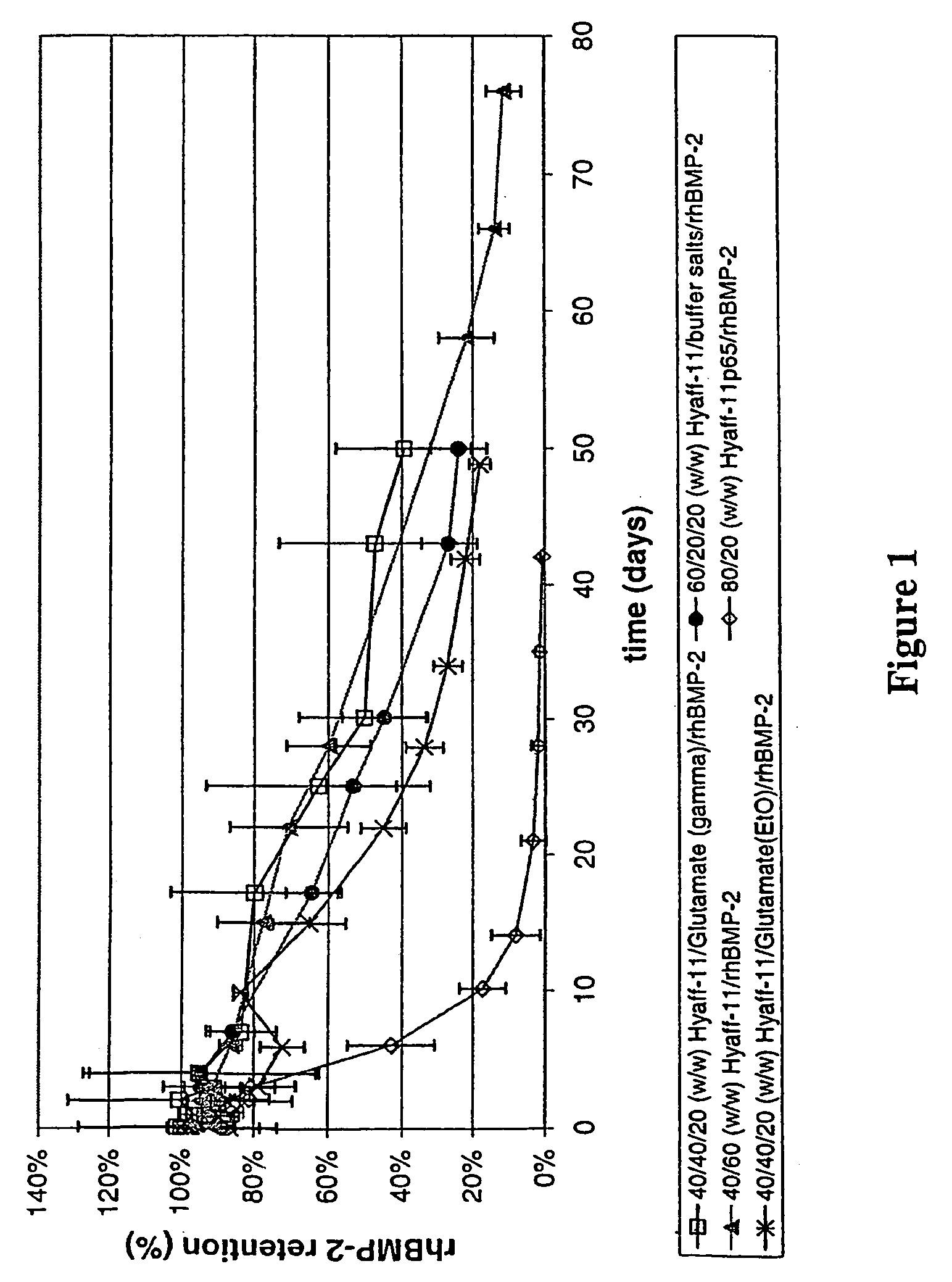
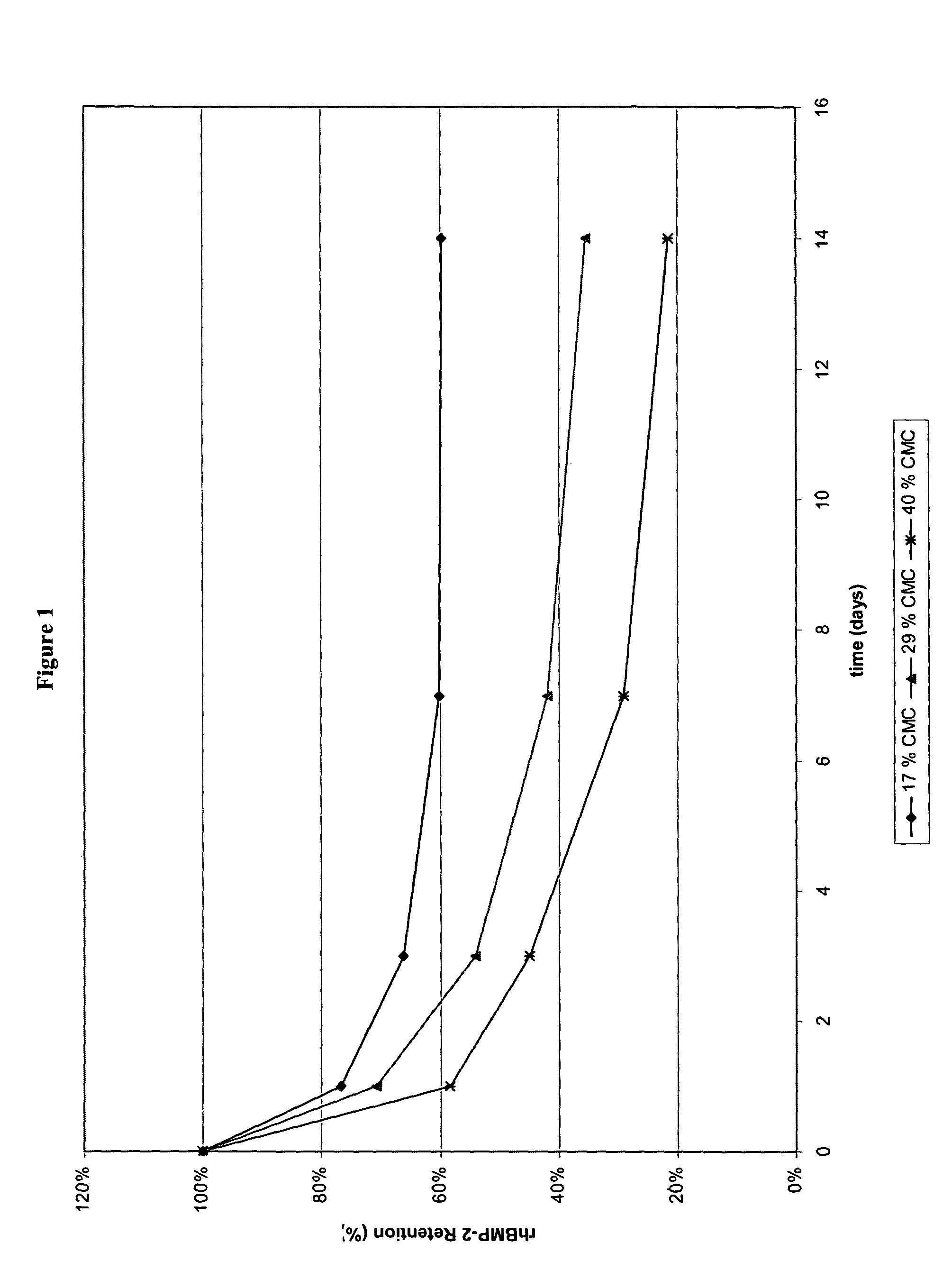
Injectable solid hyaluronic acid carriers for delivery of osteogenic proteins
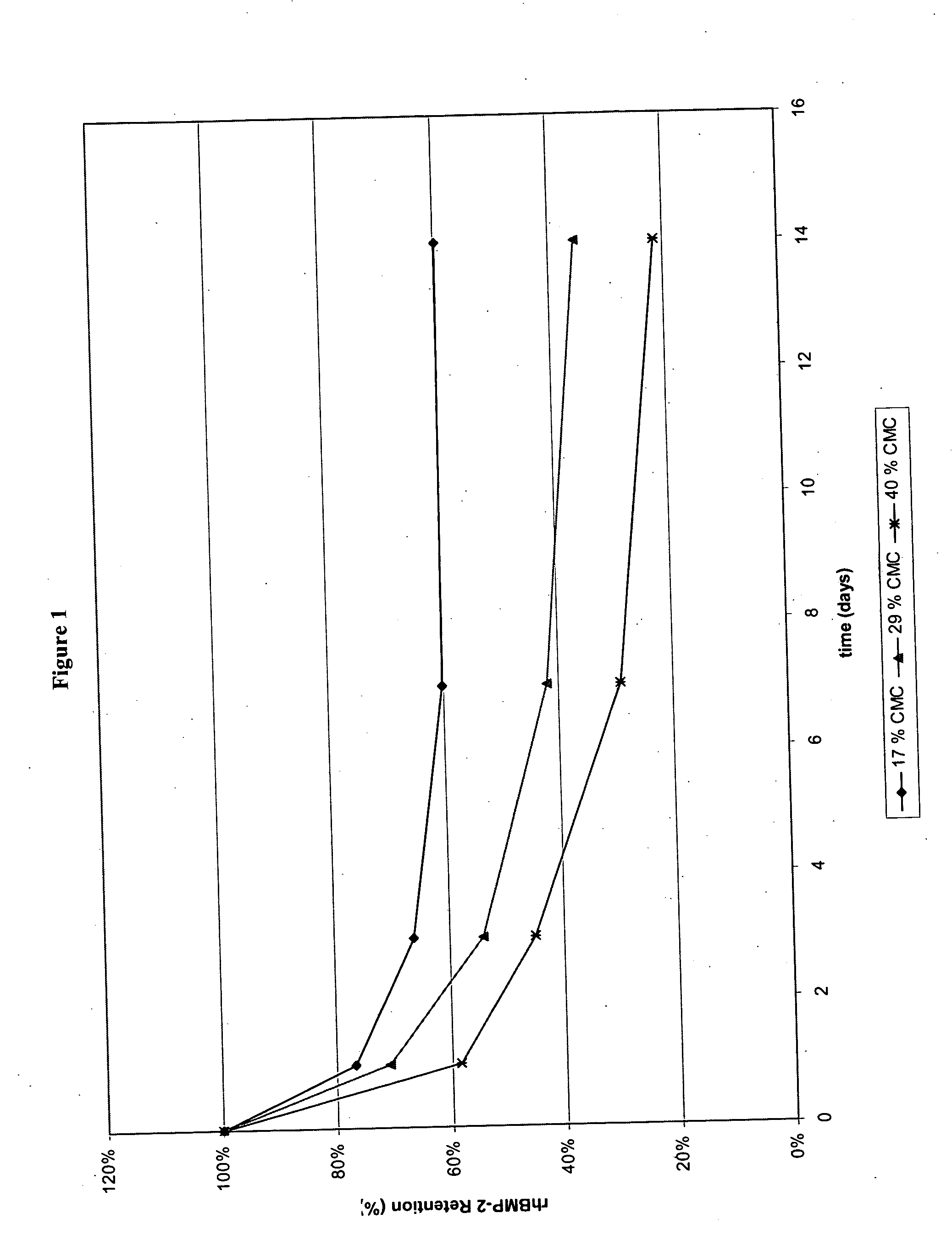
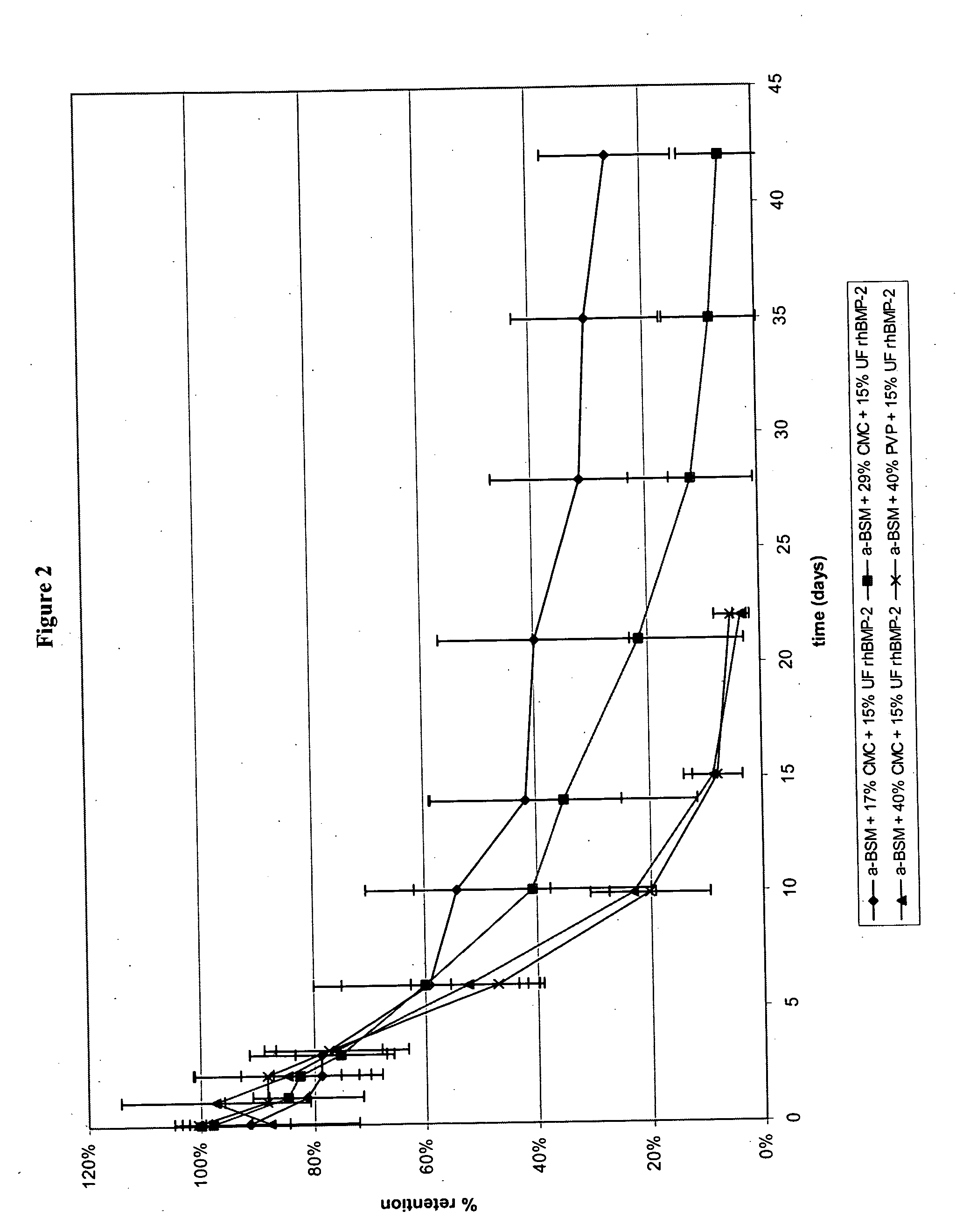
InactiveUS20090181058A1High retention rateMinimizing and reducing incidence and severityOrganic active ingredientsPeptide/protein ingredientsImplantable rodOsteogenic proteins
Methods of using an injectable or implantable rod-shaped formulation for delivery of osteogenic proteins to treat osteoporotic and / or osteopenic bone are disclosed. The formulation comprises hyaluronic acid derivatives and osteogenic proteins, and optional excipients and active ingredients such as a bone resorption inhibitor.
Owner:WYETH LLC +1
Injectable solid hyaluronic acid carriers for delivery of osteogenic proteins
InactiveUS20050287135A1High retention rateMinimizing and reducing incidence and severityBiocidePeptide/protein ingredientsImplantable rodOsteogenic proteins
An injectable or implantable rod-shaped formulation is disclosed for delivery of osteogenic proteins. The formulation comprises hyaluronic acid derivatives and osteogenic proteins, and optional excipients and active ingredients such as a bone resorption inhibitor. Methods of making injectable rod-shaped pharmaceutical compositions and methods of using the osteogenic compositions to treat osteoporotic and / or osteopenic bone are also disclosed.
Owner:WYETH +1
Cohesive osteogenic putty and materials therefor
Described is an implantable medical material comprising a malleable, cohesive, shape-retaining putty including mineral particles, insoluble collagen fibers and soluble collagen. The medical material can be used in conjunction with biologically active factors such as osteogenic proteins to treat bone or other tissue defects in patients.
Owner:WARSAW ORTHOPEDIC INC
Therapies for acute renal failure
InactiveUS20100105621A1Reduce rateImprove survival ratePeptide/protein ingredientsAntipyreticOsteogenic proteinsApoptosis
The present invention provides methods for the treatment, and pharmaceuticals for use in the treatment, of mammalian subjects in, or at risk of, acute renal failure, or subject to, or at risk of, inflammation, neutrophil-mediated cell damage, and apoptosis resulting from tissue damage or injury. The methods involve the administration of certain proteins of the osteogenic protein / bone morphogenetic protein (OP / BMP) family within the TGF-β superfamily of proteins.
Owner:STRYKER CORP
Method for the treatment of chemonucleolysis
InactiveUS7132098B2Less complicatedPromote repairPeptide/protein ingredientsBone-inducing factorOsteogenic proteinsPolysaccharide
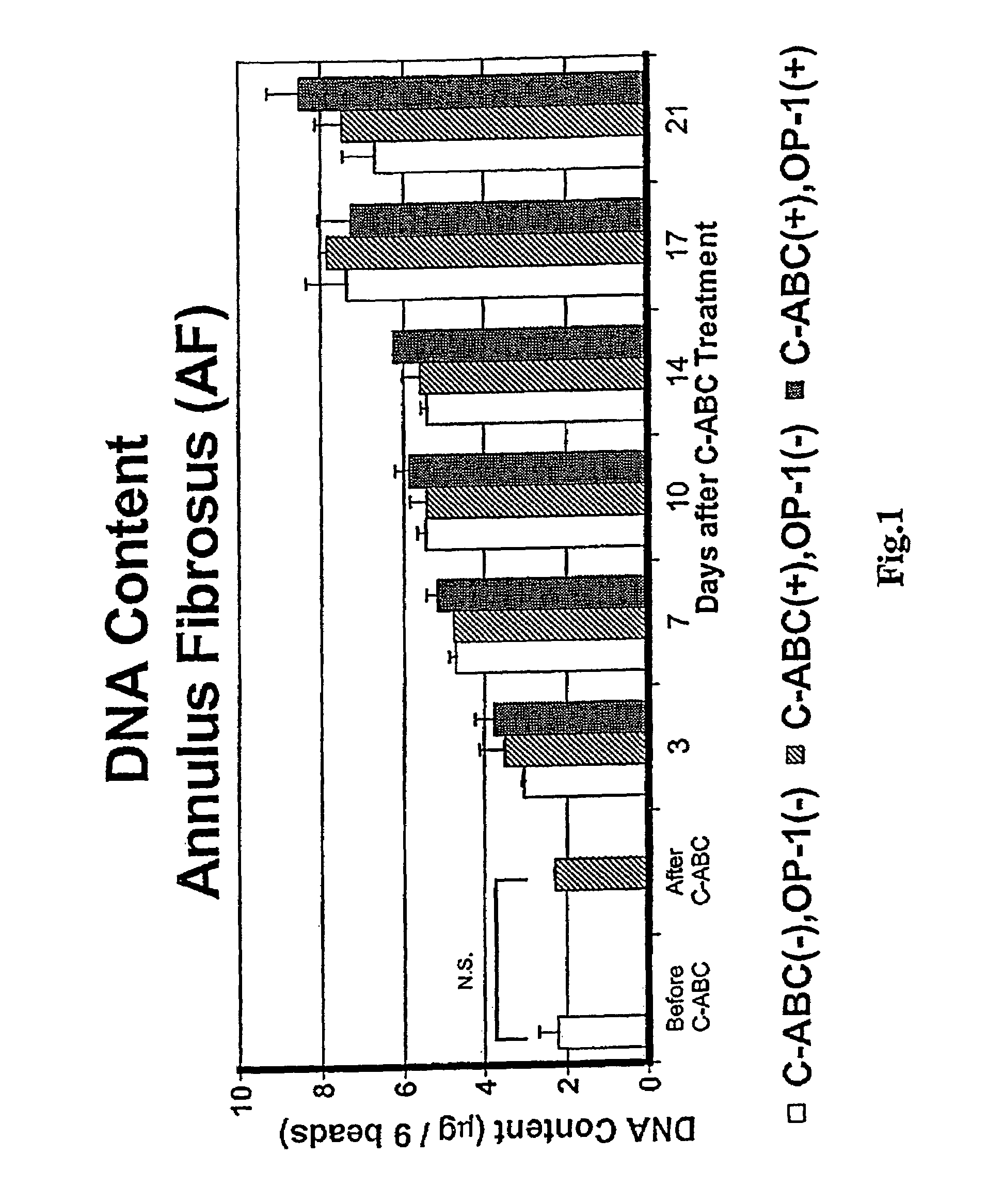
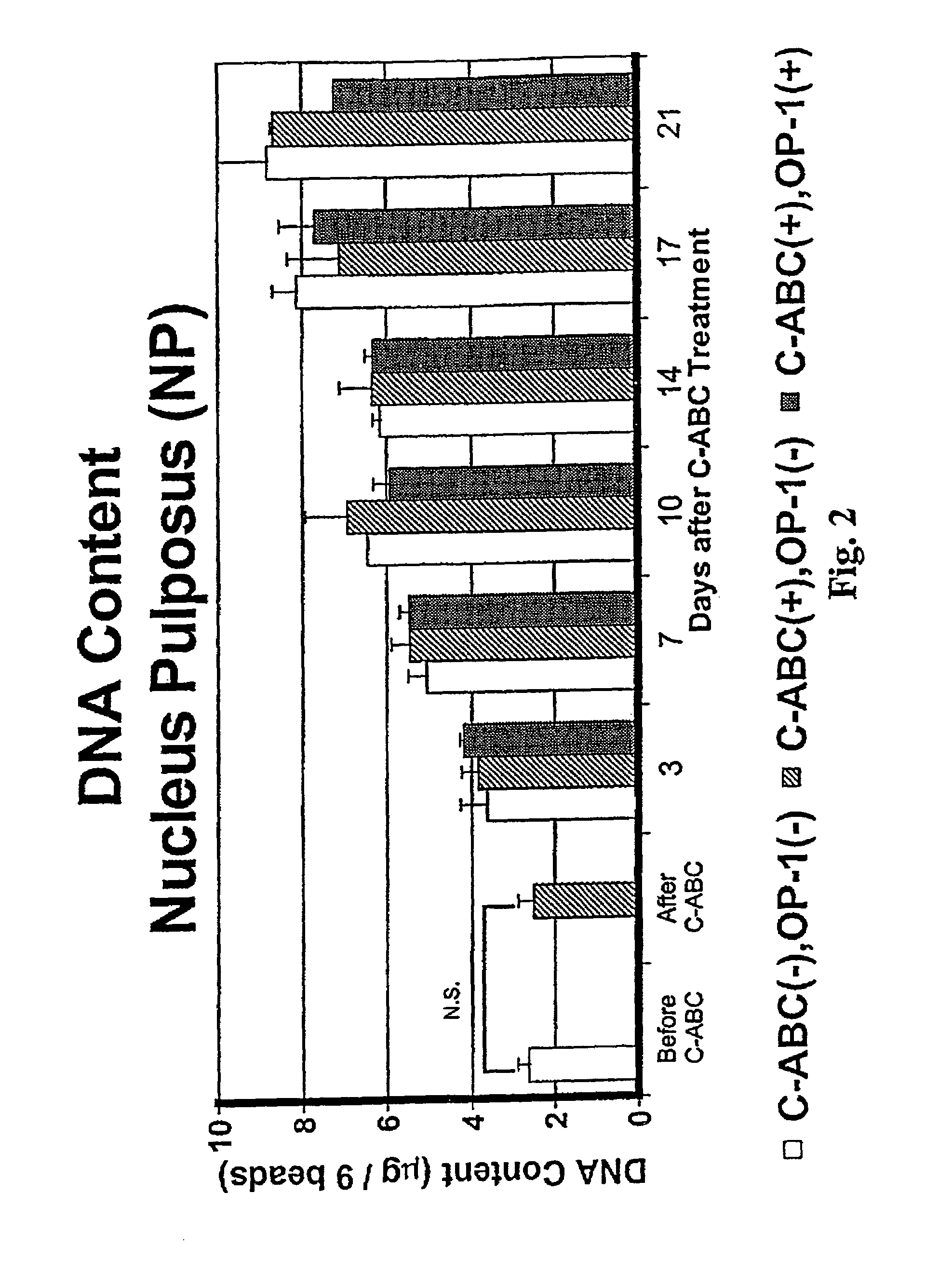
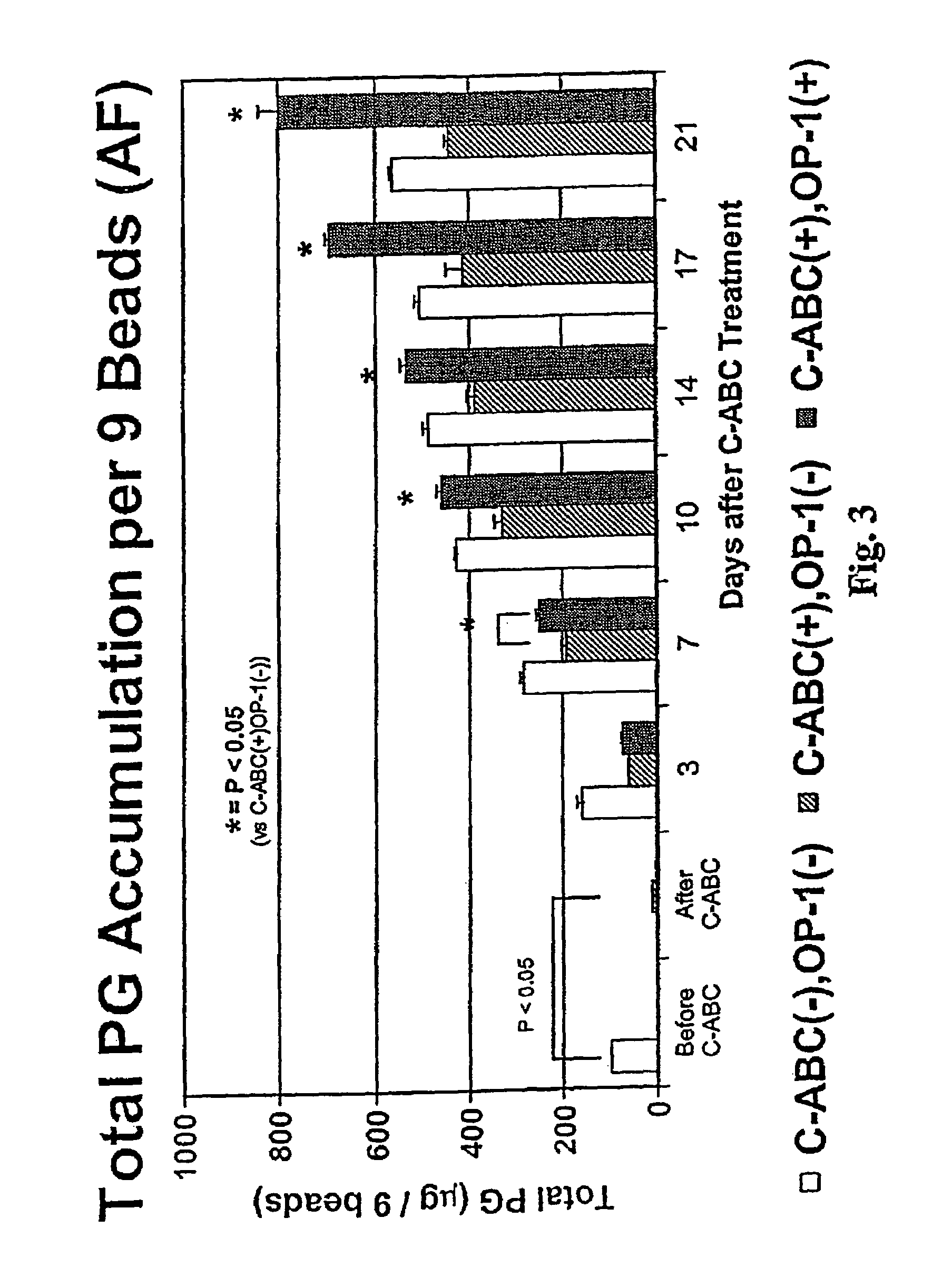
The invention relates to a method of treatment for a mammal in need of chemonucleolysis. The method comprising the administration of an effective proteoglycan cleaving amount of a proteoglycan-degrading enzyme and an effective amount of a growth factor effective in promoting the synthesis of a matrix component. The proteoglycan-degrading enzyme is preferably chondroitinase. The growth factor is preferably osteogenic protein. The proteoglycan-degrading enzyme and the growth factor are preferably administered simultaneously.
Owner:RUSH UNIV MEDICAL CENT
Injectable calcium phosphate solid rods and pastes for delivery of osteogenic proteins
Osteogenic proteins are delivered via an injectable solid rod or hardenable paste. The formulation comprises a calcium phosphate material, an osteogenic protein, and optional additives and active ingredients such as a bone resorption inhibitor. Methods of making injectable pharmaceutical compositions and methods of using the osteogenic compositions to treat bone defects are also disclosed.
Owner:ETEX +1
Osteogenic Implants with Combined Implant Materials and Methods for Same
Described are osteogenic implants that include a first implant material covered at least in part by a second implant material carrying an osteogenic protein such as a bone morphogenic protein. The first implant material can comprise a mineral and provide an inner scaffolding portion for supporting bone ingrowth, and the second implant material can comprise a collagen or other sponge carrier covering the first implant material and having a liquid osteogenic protein formulation imbibed therein. Related implant materials and methods of preparation and use constitute additional aspects of the invention.
Owner:WARSAW ORTHOPEDIC INC
Calcium phosphate delivery vehicles for osteoinductive proteins
A composition for delivery of osteogenic proteins is disclosed. The composition comprises an osteogenic protein, a calcium phosphate material as a carrier, and an effective amount of an effervescent agent. Methods of making the compositions and methods of using the osteogenic compositions to treat osteoporotic and / or osteopenic bone are also disclosed.
Owner:WYETH LLC +1
Therapies for chronic renal failure
InactiveUS7524817B2Preventing delaying needReduce frequencyOrganic active ingredientsPeptide/protein ingredientsChronic kidney failureOsteogenic proteins
Owner:MARIEL THERAPEUTICS
Methods of using bone morphogenic proteins as biomarkers for determining cartilage degeneration and aging
InactiveUS20070015178A1Microbiological testing/measurementDisease diagnosisDiseaseOsteogenic proteins
Methods are provided for determining cartilage degeneration, regeneration, or aging in a joint tissue in a patient by measuring levels of osteogenic protein-1 (OP-1) protein and / or mRNA in synovial fluid or joint tissue. The methods according to the invention are useful for detecting, diagnosing, predicting, determining a predisposition for, or monitoring joint tissue degeneration, regeneration, or aging in a patient including inflammatory joint disease or age-related disorders.
Owner:RUSH PRESBYTERIAL ST LUKES MEDICAL CENT +1
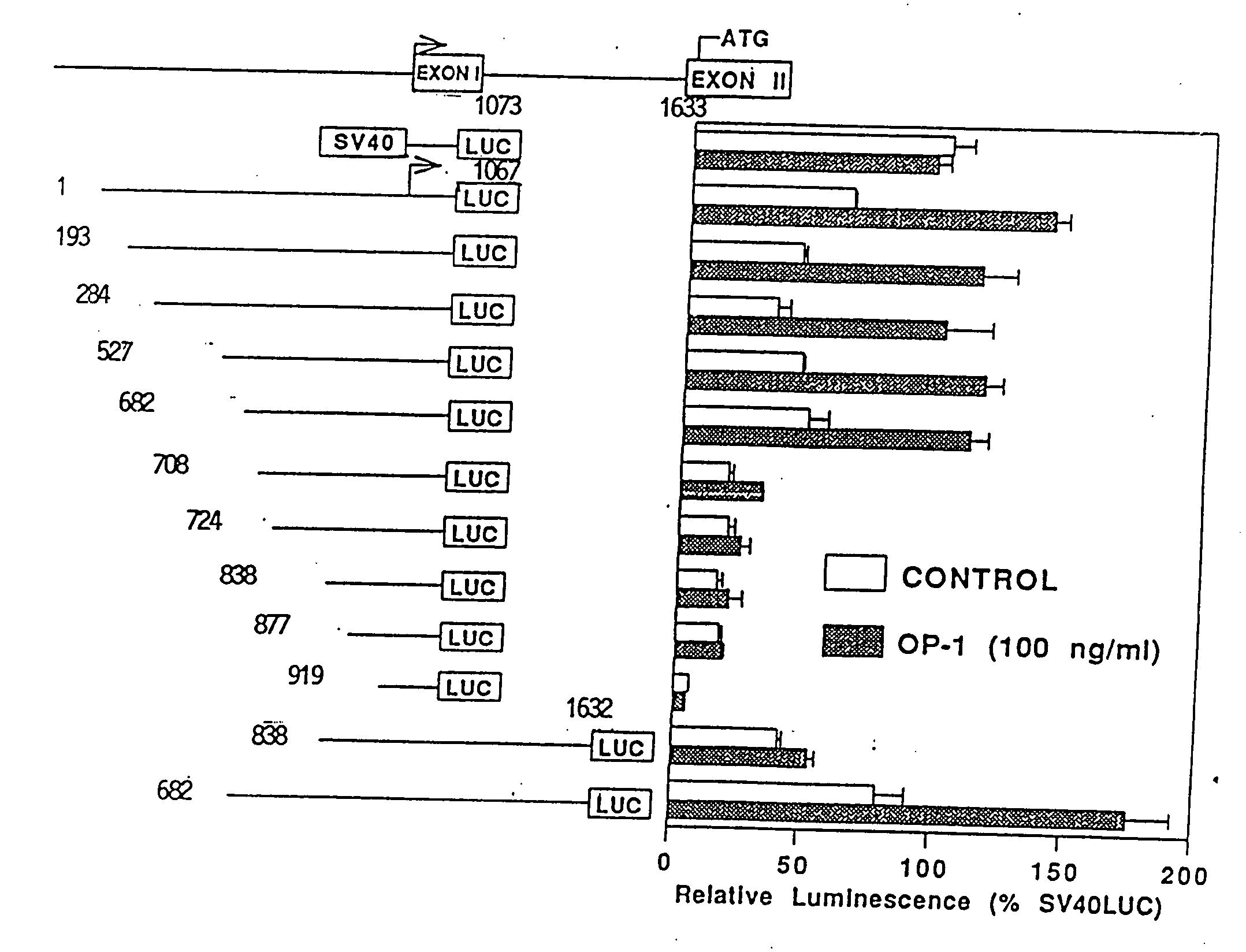
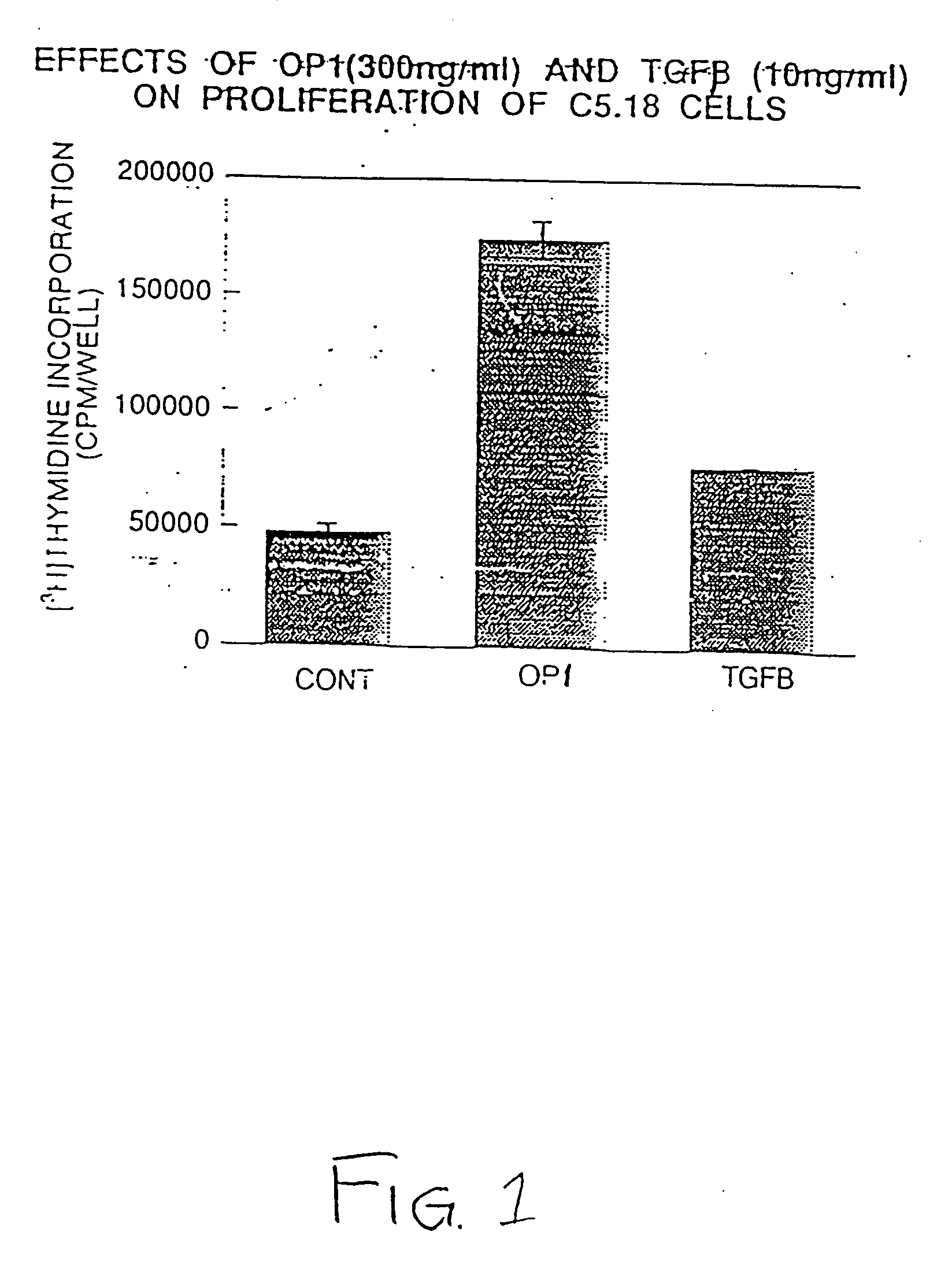
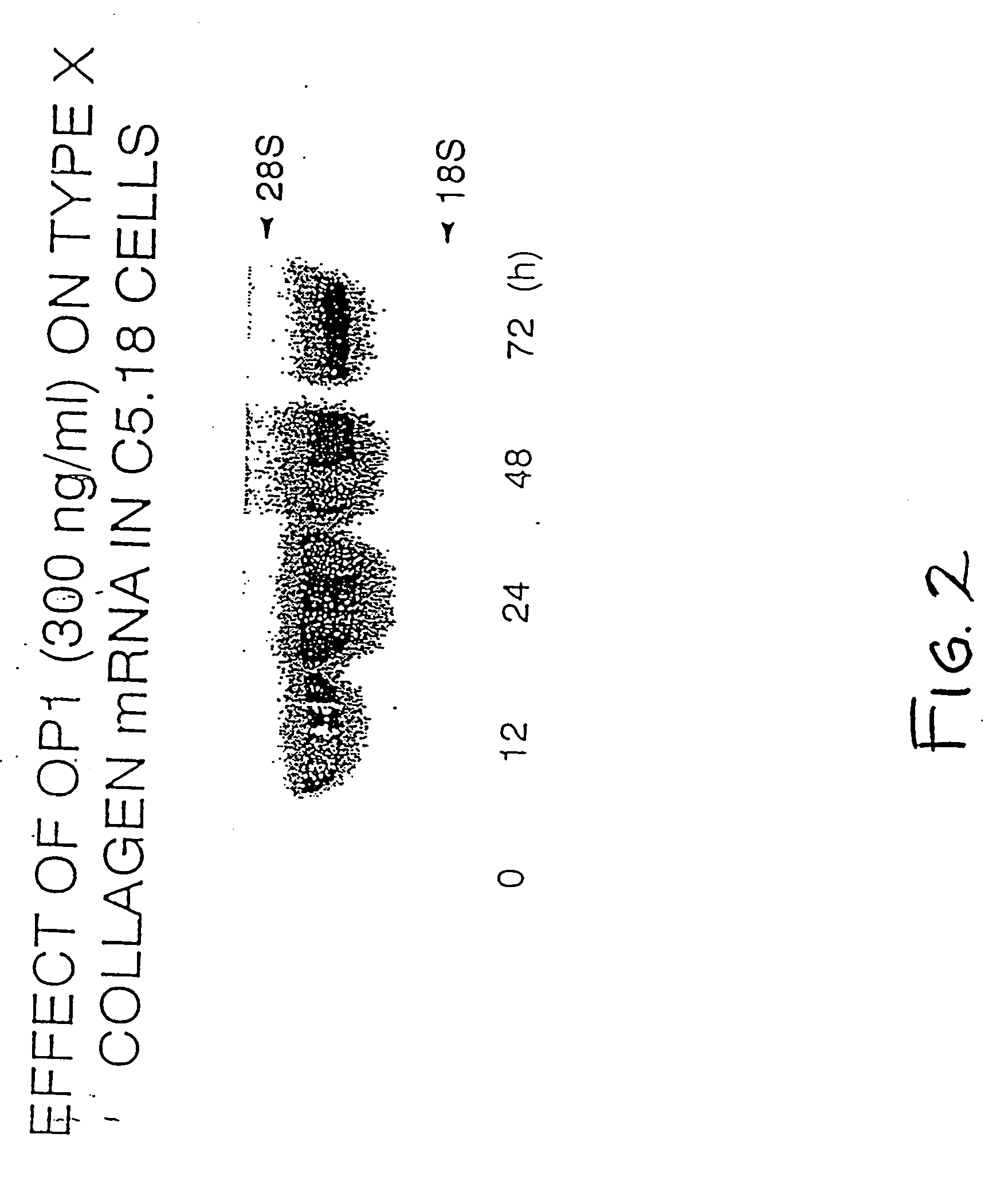
Methods and compositions for identifying morphogen analogs
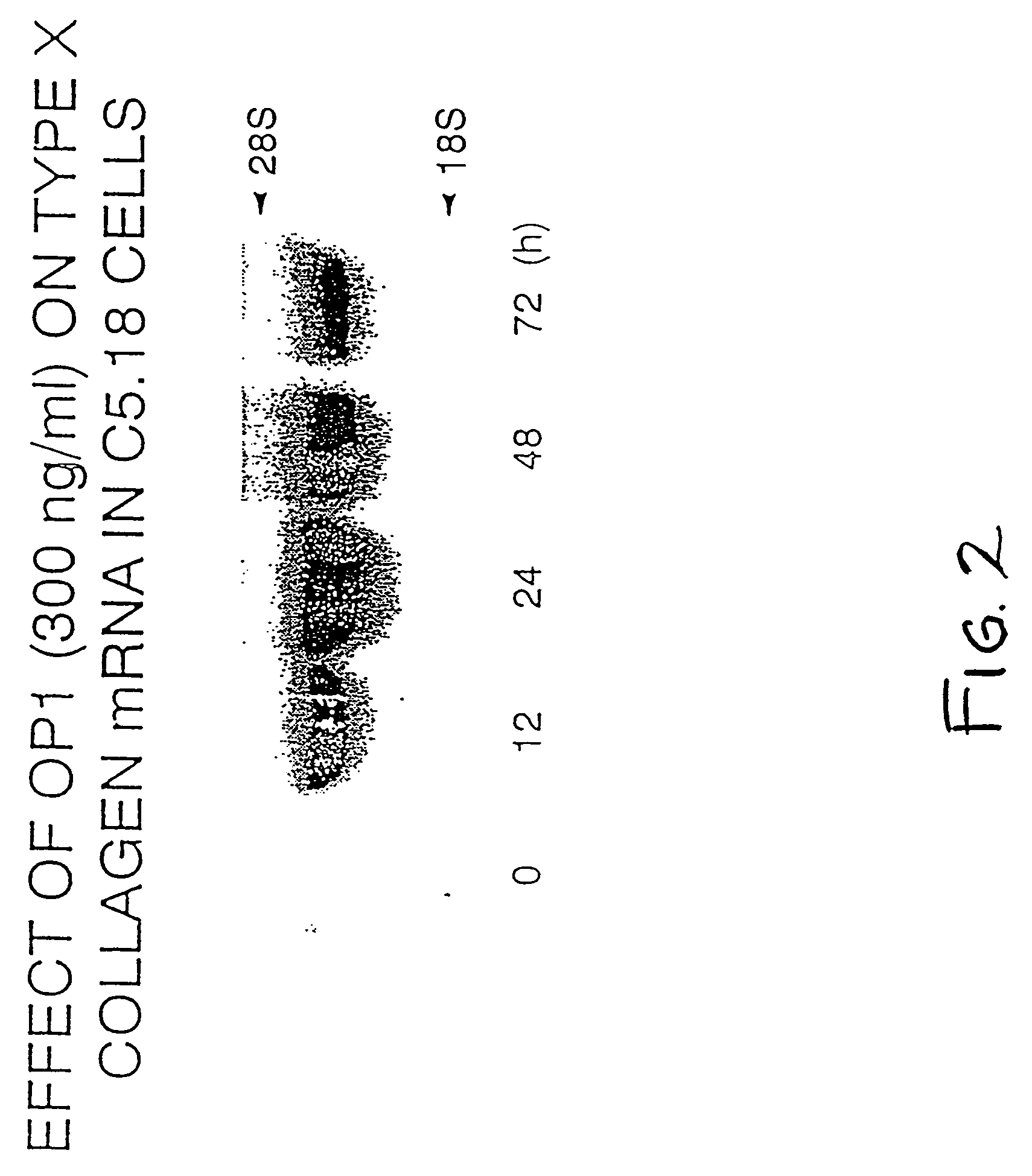
InactiveUS7306903B1Improve bindingGood for healthOrganic active ingredientsNervous disorderType X collagenMorphogen
Disclosed herein are methods and compositions for identifying morphogen analogs. The preferred methods and compositions relate to the discovery that morphogen upregulation of the mouse type X collagen promoter activity is mediated by a MEF-2 like sequence and requires an adjacent AP-1 sequence. Certain methods rest on the use of test cells comprising DNA defining a morphogen-responsive transcription activating element operatively associated with a reporter gene. Other methods rest on the use of DNAs for measuring morphogen-inducible DNA-binding. In certain preferred embodiments, the methods and DNAs involve an osteogenic protein 1 (OP-1) responsive transcription activating element. Substances that mediate interaction with and / or activate the OP-1 responsive transcription activating element are considered herein likely to be useful for reproducing in vivo effects of morphogens such as OP-1.
Owner:STRYKER CORP
Osteogenic implants with combined implant materials and methods for same
Owner:WARSAW ORTHOPEDIC INC
Osteogenic implants with combined implant materials and methods for same
ActiveUS20100266661A1Internal osteosythesisPeptide/protein ingredientsOsteogenic proteinsBone implant
Described are osteogenic implants that include a first implant material covered at least in part by a second implant material carrying an osteogenic protein such as a bone morphogenic protein. The first implant material can comprise a mineral and provide an inner scaffolding portion for supporting bone ingrowth, and the second implant material can comprise a collagen or other sponge carrier covering the first implant material and having a liquid osteogenic protein formulation imbibed therein. Related implant materials and methods of preparation and use constitute additional aspects of the invention.
Owner:WARSAW ORTHOPEDIC INC
Malleable multi-component implants and materials therefor
ActiveUS8840913B2High viscosityEfficiently formedPeptide/protein ingredientsSkeletal disorderFiberOsteogenic proteins
Described are implantable, malleable medical materials comprising mineral particles, insoluble collagen fibers, and a gel-forming polysaccharide component and / or another added gel-former. The malleable medical materials can be used treat bone or other tissue defects in patients, including in conjunction with biologically active factors such as osteogenic proteins. Also described are methods and materials that can be used to prepare the malleable medical materials.
Owner:WARSAW ORTHOPEDIC INC
Rapid Isolation of Osteoinductive Protein Mixtures from Mammalian Bone Tissue
InactiveUS20070249815A1Improved and simplifiedRapid and high and isolationPeptide/protein ingredientsBone-inducing factorBones demineralizationUltrafiltration
A method for purifying bone-derived osteogenic proteins including a demineralization process, a protein extraction process, a high molecular weight ultrafiltration process, a low molecular weight ultrafiltration process, and a recovery process. The high and low ultrafiltration processes preferably select proteins having a nominal molecular weight between approximately 8 kilodaltons and approximately 50 kilodaltons. Processes of the present invention may be used to recover osteogenic proteins from bone demineralization waste streams.
Owner:ZIMMER ORTHOBIOLOGICS
Osteogenic devices and methods of use thereof for repair of endochondral bone, osteochondral and chondral defects
InactiveUS8372805B1Restore an osteochondral or a chondral defectInhibition formationPowder deliveryPeptide/protein ingredientsRepair tissueNon union
Disclosed herein are improved osteogenic devices and methods of use thereof for repair of bone and cartilage defects. The devices and methods promote accelerated formation of repair tissue with enhanced stability using less osteogenic protein than devices in the art. Defects susceptible to repair with the instant invention include, but are not limited to: critical size defects, non-critical size defects, non-union fractures, fractures, osteochondral defects, subchondral defects, and defects resulting from degenerative diseases such as osteochondritis dessicans.
Owner:MARIEL THERAPEUTICS
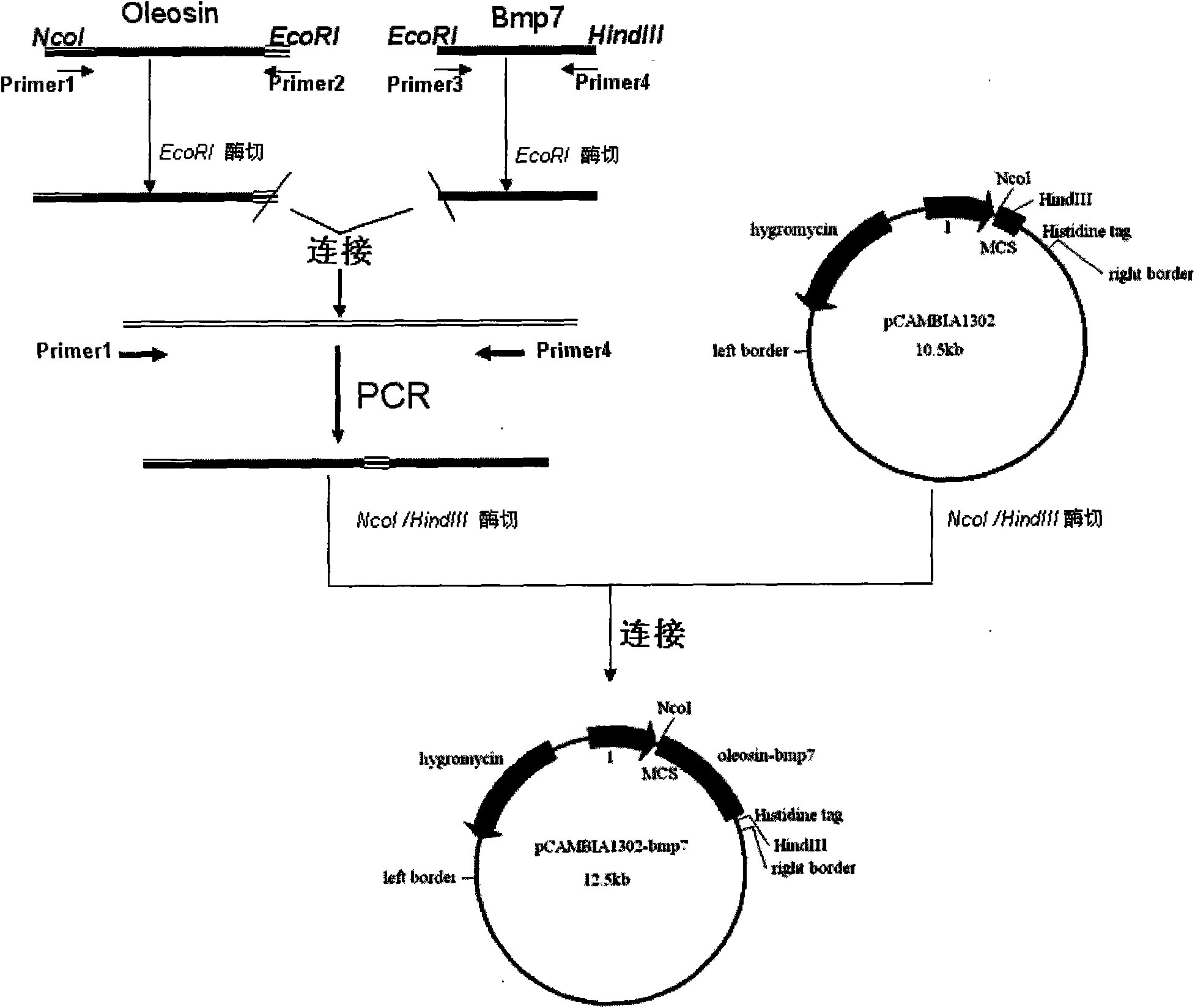
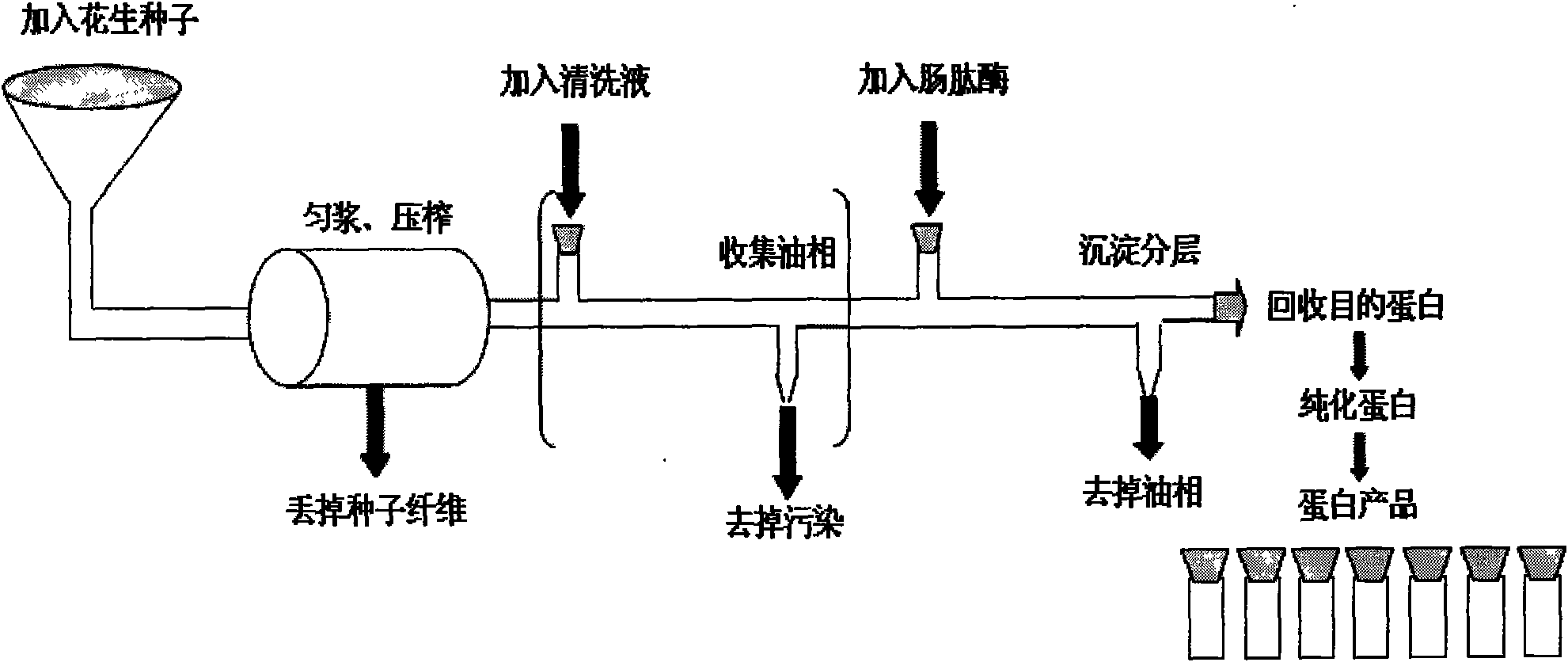
Method for expressing human osteogenic protein-1 in peanut seeds
InactiveCN101591671AHigh oil contentStable storageBone-inducing factorOsteogenic factorBiotechnologyHigh level expression
The invention discloses a method for expressing human osteogenic protein-1 in peanut seeds, which comprises the following steps of: utilizing a gene engineering way to connect an osteogenic protein encoding gene to an 3' terminal of an oleosin gene, constructing an 'oleosin-erepsin-human osteogenic protein' plant expression vector driven by an oleosin promoter, transforming peanuts, making recombinant protein perform high-level expression in the peanut seeds along with the accumulation of an oil body, separating and purifying the recombinant protein through floating centrifugation, and then purifying to obtain the protein after the digestion of exoproteinase. The recombinant protein obtained by the method has the advantages of safety, high activity, and easy recovery and purification, can be stored in the peanut seeds for a long time, has convenient transportation, and can increase the added value of farm products.
Owner:山东省农业科学院高新技术研究中心
Methods and compositions for identifying morphogen analogs
InactiveUS20080003675A1Improve bindingGood for healthOrganic active ingredientsNervous disorderType X collagenMorphogen
Disclosed herein are methods and compositions for identifying morphogen analogs. The preferred methods and compositions relate to the discovery that morphogen upregulation of the mouse type X collagen promoter activity is mediated by a MEF-2 like sequence and requires an adjacent AP-1 sequence. Certain methods rest on the use of test cells comprising DNA defining a morphogen-responsive transcription activating element operatively associated with a reporter gene. Other methods rest on the use of DNAs for measuring morphogen-inducible DNA-binding. In certain preferred embodiments, the methods and DNAs involve an osteogenic protein 1 (OP-1) responsive transcription activating element. Substances that mediate interaction with and / or activate the OP-1 responsive transcription activating element are considered herein likely to be useful for reproducing in vivo effects of morphogens such as OP-1.
Owner:STRYKER CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com