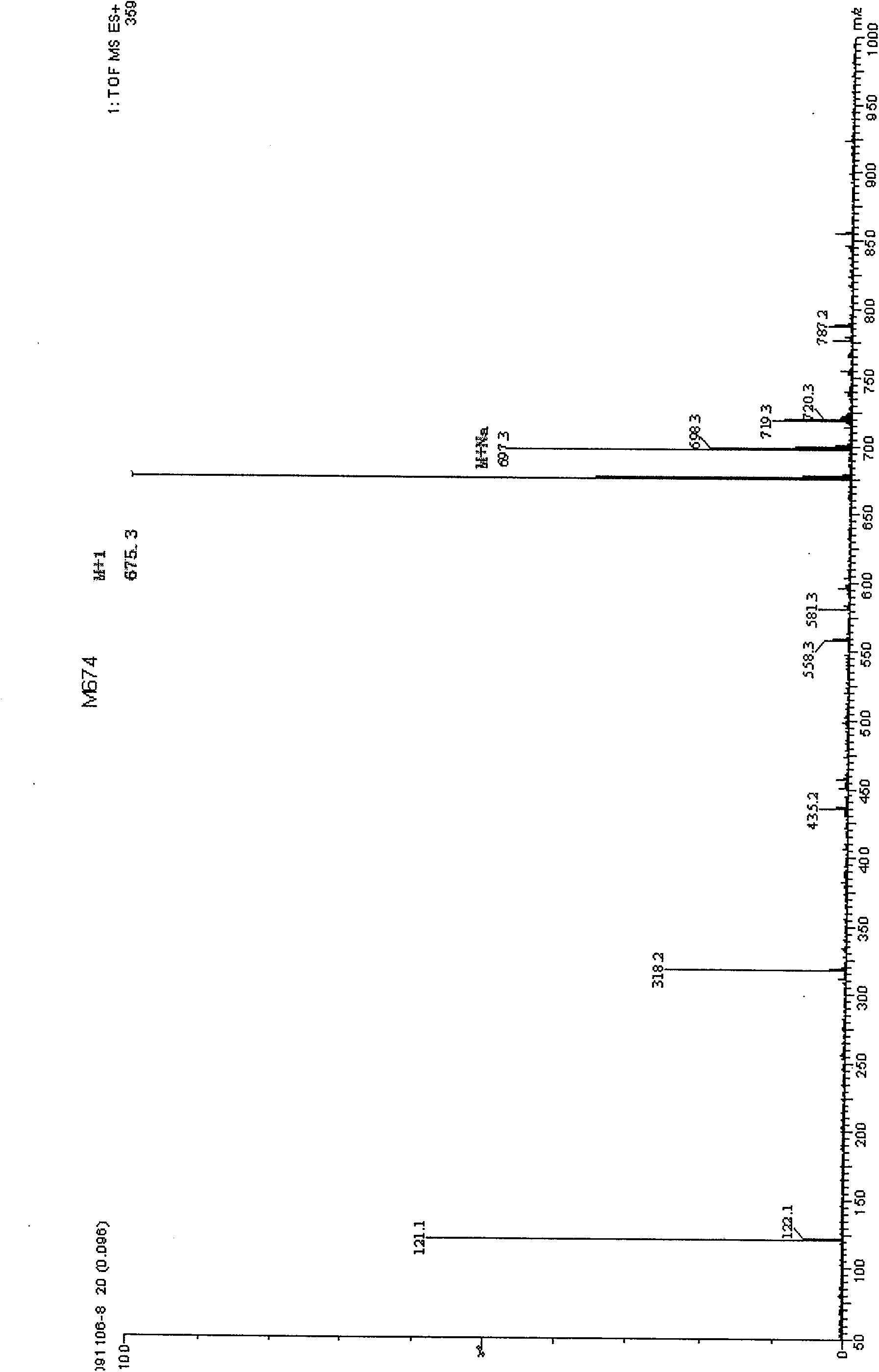
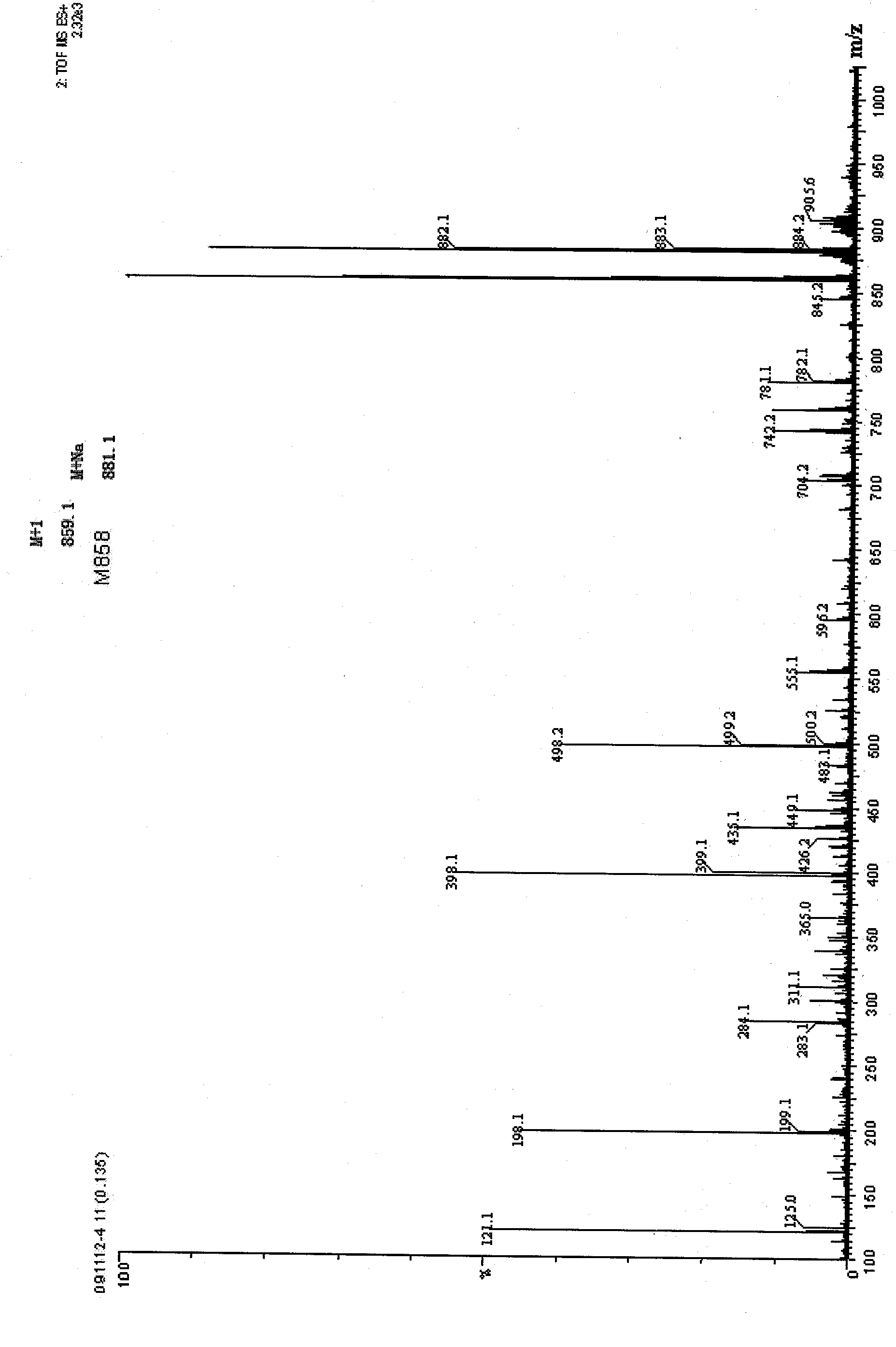
Nucleoside disulfide dinitrogen derivative and preparation method thereof
A technology of nucleoside disulfide and derivatives, which is applied in the field of synthesis of radiopharmaceutical-labeled precursor compounds, and can solve problems such as no relevant reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
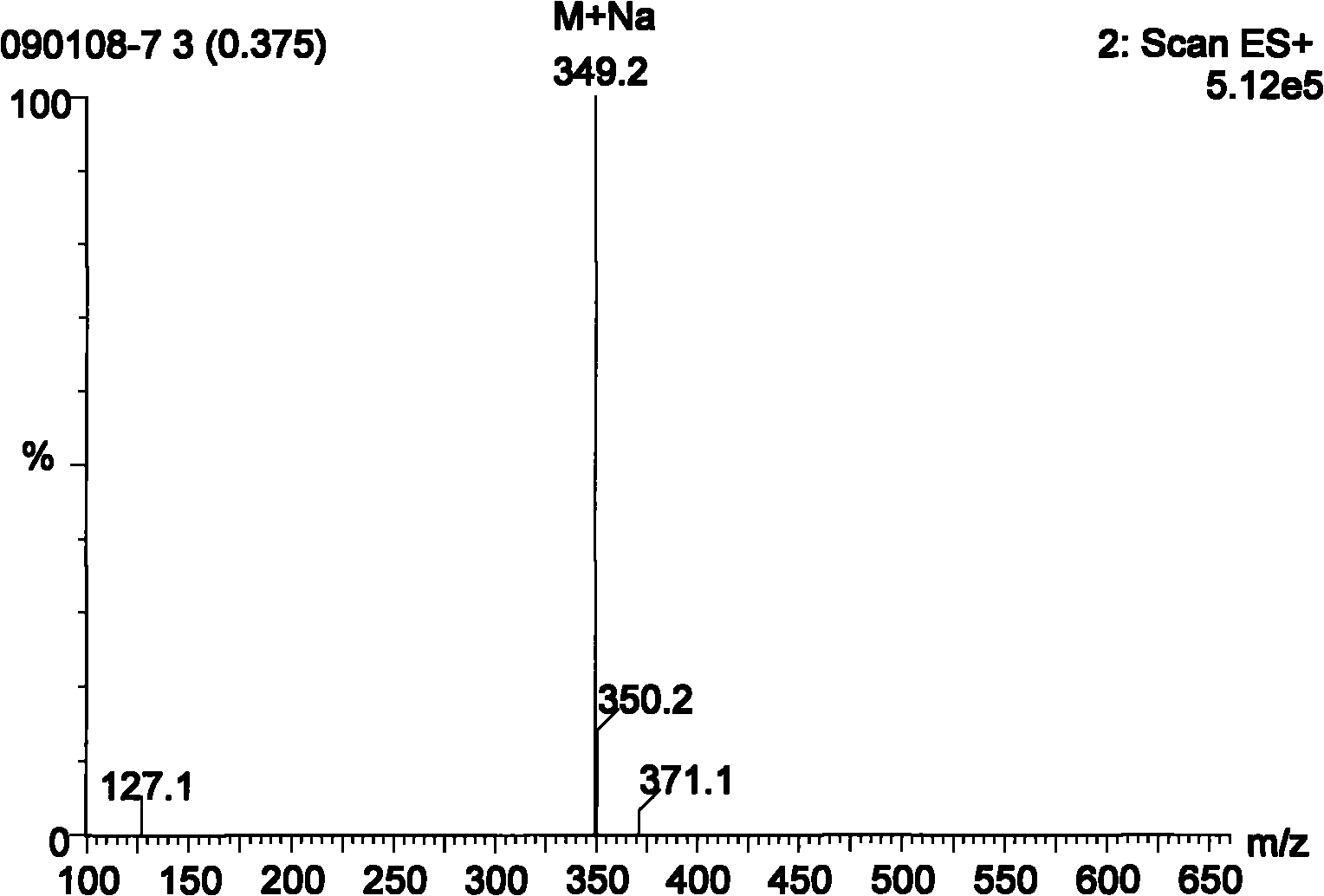
[0032] Example 1 3', the synthesis of 5'-diacetylthymidine (product 2):
[0033] Take β-thymidine (2g, 8.26mmol), DMAP (0.1g) and join in 80mL dichloromethane solution, under the condition of ice-water bath, add dropwise the dichloromethane solution containing acetic anhydride (8mL) 20mL, dropwise finish, react The temperature of the solution was raised to 40°C, and the stirring was continued until the reaction solution was clear, and saturated NaHCO 3 Neutralize the excess acetic anhydride in the solution until no bubbles are produced, wash the organic phase with a large amount of water, anhydrous Na 2 SO 4 Dry, concentrate, pass through a silica gel column, the eluent is: ethyl acetate / petroleum ether=4 / 1 (v / v), the product obtained is recrystallized with ethyl acetate / petroleum ether to obtain 2.5 g of white crystals, the yield It is 92.84%, and the melting point is 124-126°C. Mass spectrum Ms (m / z): 349 (M+Na); 1 HNMR: CH at the 5th position of the pyrimidine ring 3 -...
Embodiment 2
[0034] Example 2 N 3 Synthesis of -tert-butoxycarbonyl-3',5'-diacetylthymidine (product 3):
[0035]Take 1.95g (5.98mmol) of product 2, dissolve it in 20mL of anhydrous tetrahydrofuran THF, add 2.7g (12.38mmol) of tert-butoxyformic anhydride, stir at room temperature for 80min, then add 4g (32.79mmol) of DMAP, and continue stirring at room temperature After 4 hours, evaporate the solvent to dryness, add 80 mL of ethyl acetate, then wash with water and dilute acetic acid respectively, dry the organic phase, evaporate to dryness and pass through the column, the eluent is ethyl acetate / petroleum ether=1 / 1 (v / v ), to obtain 2.5 g of white foamy solid, with a yield of 98.13%. Mass spectrum Ms (m / z): 449 (M+Na), 875 (2M+Na); 1 HNMR: Three CHs on tert-butyl 3 -1.55ppm (9H, singlet), CH at the 5th position of the pyrimidine ring 3 -1.88ppm (3H, single peak), CH of two acetyl groups 3 -2.105ppm (6H, singlet), CH at the 2-position of the pentose ring 2 -2.41ppm (2H, multiplet), CH...
Embodiment 3
[0036] Example 3 N 3 Synthesis of -tert-butoxycarbonyl-3',5'-bisacetyl-5-bromomethyl-2'-deoxyuridine (product 4):
[0037] Under the protection of nitrogen, the product 3 (1.0g, 2.3mmol) and a small amount of BPO (15mg) were dissolved in 100mL of dry carbon tetrachloride, and under light conditions, 0.38g (2.3mmol) of Br 2 Carbon tetrachloride solution 20mL, reflux reaction 3h, the temperature of the reaction solution is lowered to room temperature, carbon tetrachloride is evaporated to dryness by rotary evaporation, and the residue is passed through a silica gel column, and the eluent is ethyl acetate / petroleum ether=1 / 2(v / v) to obtain 0.48 g of white foamy solid, with a yield of 41.32%. Mass spectrum Ms (m / z): 527, 529 (M+Na); 1 HNMR: Three CHs on tert-butyl 3 -1.62ppm (9H, singlet), one acetyl CH 3 -2.08ppm (3H, singlet), one acetyl CH 3 -2.12ppm (3H, singlet), CH at the 2-position of the pentose ring 2 -2.41ppm (2H, multiplet), CH- at the 3rd position and CH at the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com