Fluorescence detection method of five-position aldehyde-group deoxidizing uridine
An aldehyde-based deoxyuridine and detection reagent technology, applied in fluorescence/phosphorescence, material excitation analysis, etc., can solve the problems of large required amount, complex cost, weak selectivity, etc., and achieve the effect of strong selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: 4-methoxy o-phenylenediamine was used to react with deoxycytidine, five-aldeoxycytidine and five-aldeoxyuridine respectively and measure the fluorescence spectrum thereof.
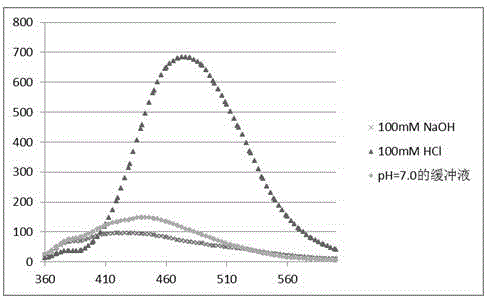
[0027] 4-Methoxy-o-phenylenediamine, penta-aldeoxyuridine and dithiothreitol were prepared as 10 mM solutions in DMSO (dimethyl sulfoxide). Then dilute 10mM 4-methoxy-o-phenylenediamine to 1mM solution for later use, and prepare acetic acid buffer solution with pH=4.5 with ammonium acetate and acetic acid at a concentration of 1M.
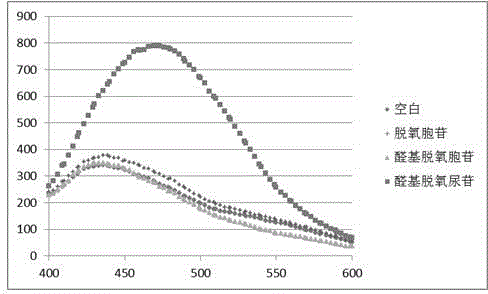
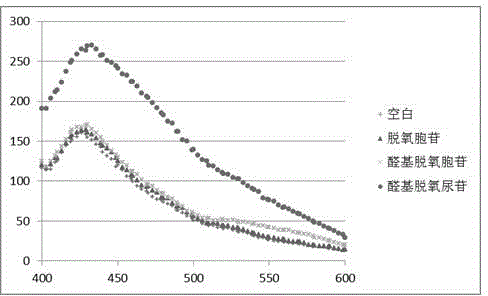
[0028] Take 8 μL of 1 mM 4-methoxy-o-phenylenediamine solution, 4 μL of 10 mM 10 mM dithiothreitol solution, 4 μL of 10 mM penta-aldehyde deoxyuridine solution, 4 μL of 1M acetate buffer, and add 380 μL of high-purity water to prepare into 400 μL mixed solution and detect its fluorescence spectrum after reacting for 3 hours, a new peak appeared at 475nm, compared with Figure three The peak of compound A in ? confirms the occurrence of the reaction.
[0029]...
Embodiment 2
[0034] Embodiment 2: Use o-phenylenediamine to react with deoxycytidine, five-formyl deoxycytidine and five-formyl deoxyuridine respectively and measure its fluorescence spectrum
[0035] O-phenylenediamine, penta-aldeoxyuridine and dithiothreitol were prepared into 10 mM solutions in DMSO (dimethyl sulfoxide). Then dilute the 10mM o-phenylenediamine into 1mM solution for later use, prepare the acetate buffer solution with pH=4.5 with ammonium acetate and acetic acid, the concentration is 1M.
[0036] Take 8 μL of 1 mM o-phenylenediamine solution, 4 μL of 10 mM 10 mM dithiothreitol solution, 4 μL of 10 mM penta-aldehyde deoxyuridine solution, 4 μL of 1M acetate buffer, add 380 μL of high-purity water to prepare a 400 μL mixture and mix Detect its fluorescence spectrum after reacting for 3 hours, new peak appears at 430nm place, contrast Figure four The peak of compound B in ? confirms the occurrence of the reaction.
[0037] O-phenylenediamine, penta-aldeoxycytidine and dit...
Embodiment 3
[0042] Embodiment 3: the chemical synthesis of compound A
[0043] Dissolve 17mg of 4-methoxy-o-phenylenediamine and 30mg of penta-aldehyde deoxyuridine in 2mL of DMF solvent. After stirring at room temperature for one hour, add 6mg of catalyst scandium trifluoromethanesulfonate and 12μL of oxidant hydrogen peroxide (30%) , and exposed to the air to react. After the reaction was detected by TLC, the solvent was evaporated under reduced pressure, and the column was purified with eluent (chloroform:methanol=20:1) to obtain 29.8mg of compound A, the yield was 68%, and it was brown yellow solid, 1 H NMR (300MHz DMSO-d 6 ) δ 2.213 (s, 2H), 3.596 (s, 2H), 3.744 (s, 3H), 3.856 (s, 1H), 4.269 (s, 1H), 5.061 (s, 1H), 5.331 (s, 1H) , 6.197 (t, J=6.3Hz, 1H), 6.755 (d, J=8.4Hz, 1H), 7.093 (d, J=19.2Hz, 1H), 7.439 (t, J=8.1Hz, 1H), 8.743 (d, J=12Hz, 1H), 11.910 (s, 1H), 12.021 (s, 1H); 13 C NMR (300MHz DMSO-d 6 ) δ 19.222, 55.990, 56.083, 56.701, 62.069, 71.168,71.225,85.834,88.429,88...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com