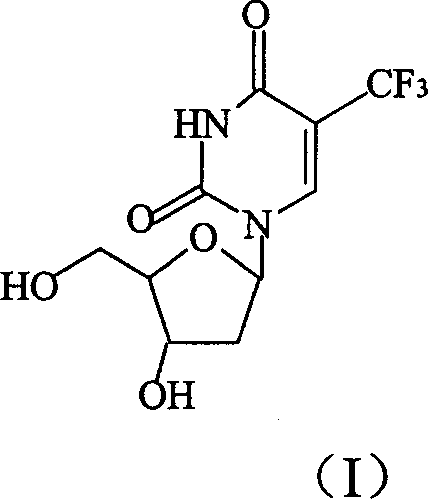
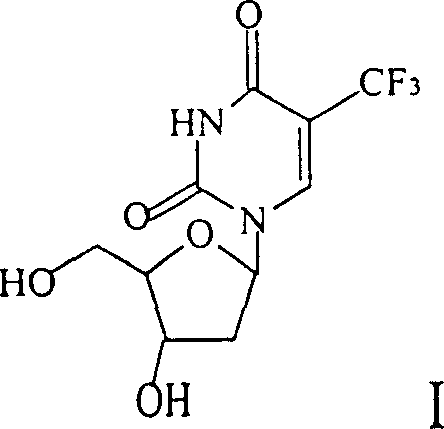
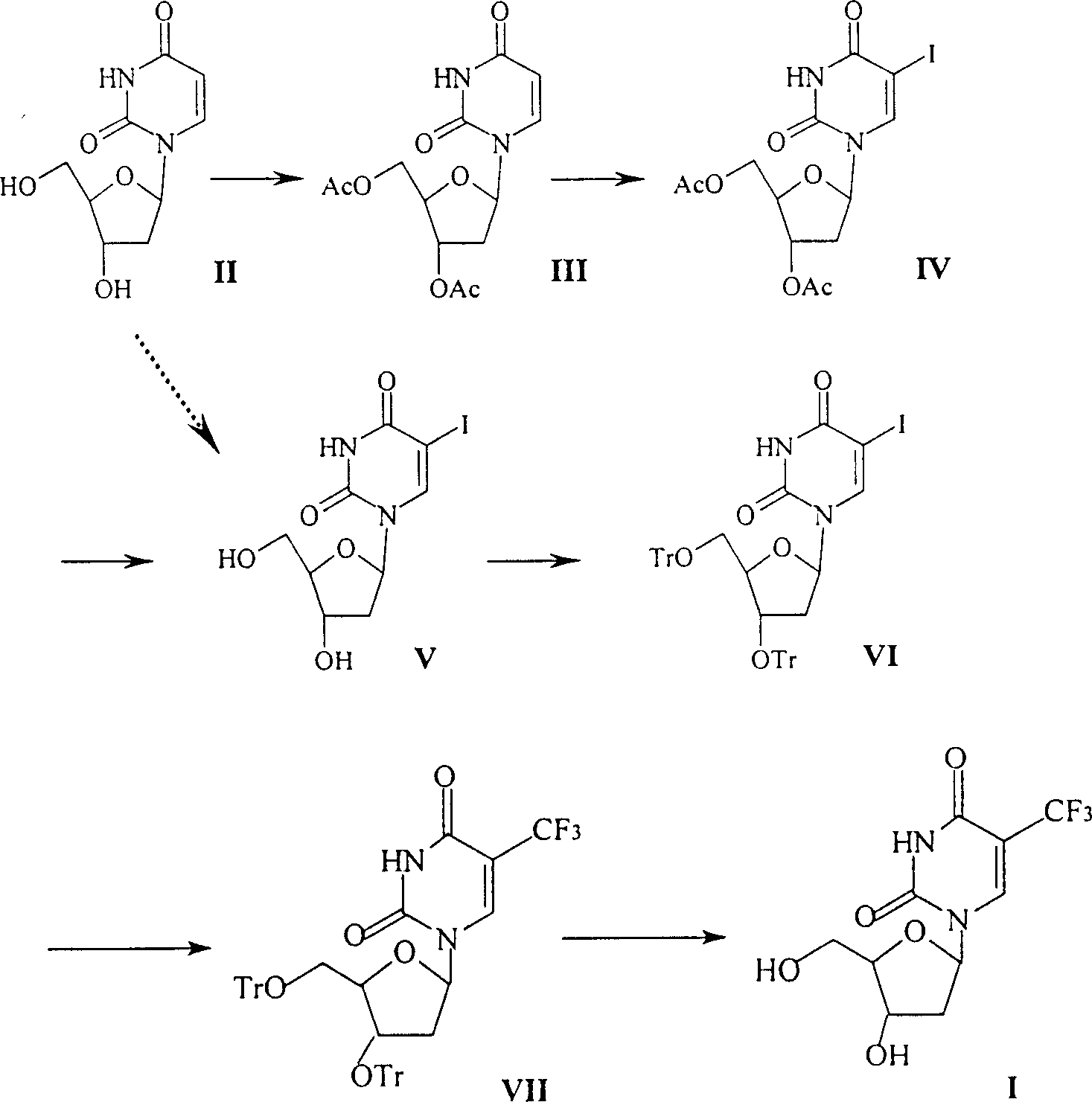
Method for synthesizing 5-trifluoro methyl-2'-desugarized uridine
A technology of deoxyuridine and trifluoromethyl, applied in sugar derivatives, organic chemistry, etc., can solve the problems of easy pollution of the environment, and achieve the effect of simple operation, easy availability of synthetic raw materials, and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1 3', the preparation of 5'-diacetyl-2'-deoxyuridine (III)
[0017] 2'-deoxyuridine (1.03 g, 4.5 mmol), acetic anhydride (12 ml) and 4-N, N-dimethylaminopyridine (DMAP) (0.4 g) were added to the reaction flask, and the mixture was stirred at room temperature overnight . Concentrate to dryness under reduced pressure, add water to the obtained colorless solid, and filter to obtain 3',5'-diacetyl-2'-deoxyuridine product (1.23 g), yield 88%, mp108-109 ° C; [ α] D 25 15.5 (c1.2, CHCl 3 ).
[0018] 1 H NMR (δ, ppm, CDCl 3 ): 2.14 (s, 6, 3’ and 5’-COCH 3 ), 2.16-2.57(m, 2, H-2', H-2"), 4.35(m, 3, H-4', H-5', H-5"), 5.14-5.38(m, 1 , H-3'), 5.80(d, J=8.0Hz, 1, H-5), 6.30(d of d, J=8and 6Hz, 1, H-1'), 7.52(d, J=8.0Hz , 1, H-6).
Embodiment 2
[0019] Example 2 Preparation of 3', 5'-diacetyl-2'-deoxyuridine (III)
[0020] The preparation method is the same as in Example 1, wherein pyridine is used instead of 4-N, N-dimethylaminopyridine, and the reaction time is 2-3 days.
Embodiment 3
[0021] Example 3 Preparation of 5-iodo-3', 5'-diacetyl-2'-deoxyuridine (IV) 3', 5'-diacetyl-2'-deoxyuridine (0.5mmol), iodine (0.6 mmol), CAN (0.25 mmol) and acetonitrile (8 mL) was stirred at 80°C for one hour. Evaporate the solvent under reduced pressure, add saturated aqueous sodium chloride solution (10 ml) and a small amount of sodium bisulfite aqueous solution to the residue, extract three times with ethyl acetate (10 ml × 3), combine the organic phases, wash with water, and It was washed with aqueous solution (15 ml) and water (15 ml×2), and dried over anhydrous magnesium sulfate. After filtration, the filtrate was evaporated to dryness, and the crude product was purified by silica gel column chromatography or recrystallized from ethanol to obtain 201 mg of a colorless needle product with a melting point of 160.5-162°C.
[0022] 1 H NMR (δ, ppm, CDCl 3 ): 2.07, 2.11 (2s, 3 and 3, 3’ and 5’-COCH 3 ), 2.29-2.48(m, 2, H-2', H-2"), 4.20-4.29(m, 3, H-4', H-5', H-5"), 5.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com