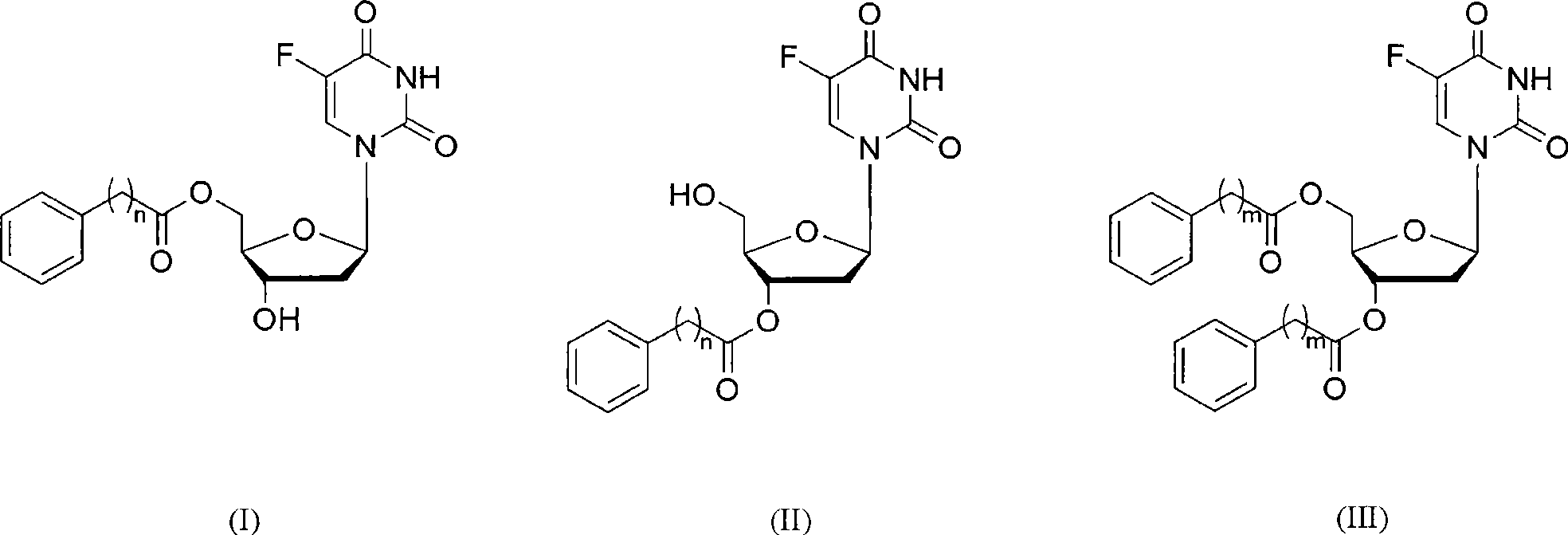
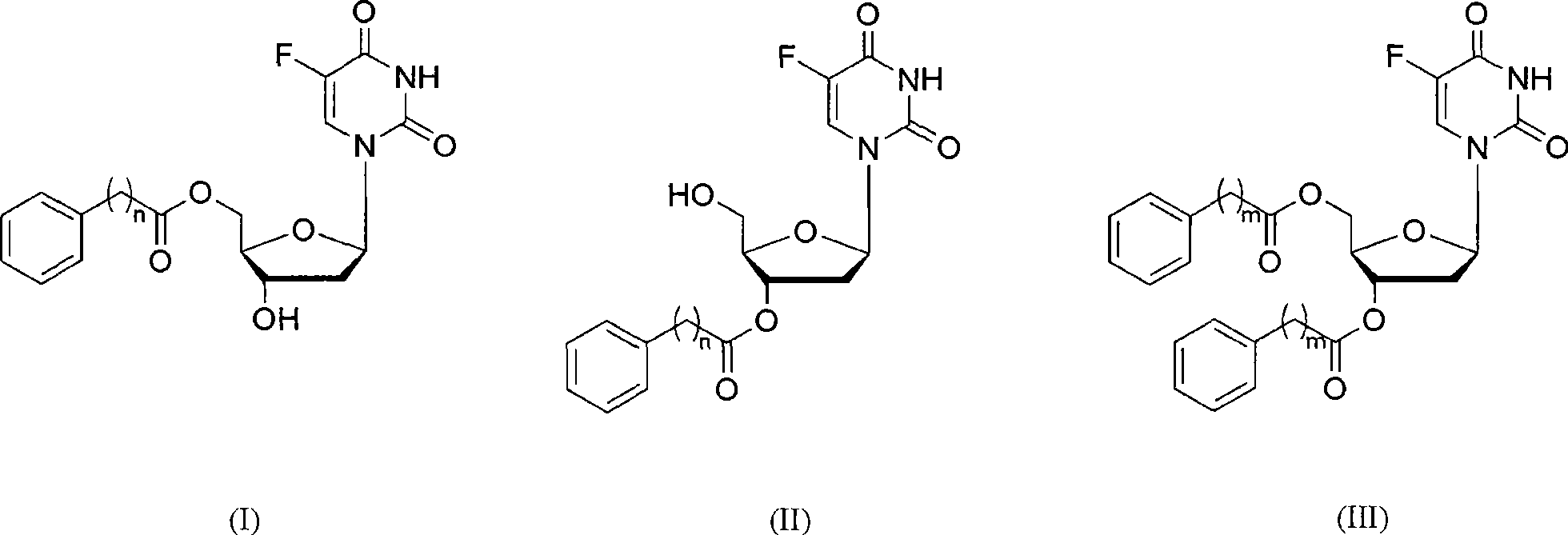
Fragrant acid ester derivative of 5-fluorine-2'-deoxidized uridine and synthesis thereof
A technology of deoxyuridine arylate and synthesis method, which is applied in the direction of sugar derivatives, drug combination, organic chemistry, etc., can solve the problems of low regioselectivity, difficult product separation, and many reaction steps, and achieve the goal of overcoming low selectivity , The reaction process is simple and easy to control, and the product is easy to produce
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Synthesis of 3'-phenylacetate of 5-fluoro-2'-deoxyuridine
[0027] Add 5-fluoro-2'-deoxyuridine (101mg, 0.41mmol), vinyl phenylacetate (1.64mmol), and 30mL tetrahydrofuran into a sealed Erlenmeyer flask, followed by adding 200mg of Burkholderia The immobilized lipase of cepacia) was shaken in a constant temperature oscillator at 50° C. and 200 rpm under normal pressure, and the reaction was monitored by TLC. After the reaction, filter, concentrate the filtrate under vacuum, and finally purify by column chromatography to obtain 134 mg of the product, the yield is 90%, white powder, and the purity is greater than 99%. The Rf value and NMR data of 3'-phenylacetate of 5-fluoro-2'-deoxyuridine on TLC are as follows: Rf=0.31 (PE / EA=1 / 1); 1 H NMR (DMSO-d 6 )δ: 2.27 (apparent d, 2H, H 2′ ), 3.64(s, 2H, H 2″ ), 3.74(s, 2H, H 5′ ), 4.02(s, 1H, H 4′ ), 5.26(s, 1H, H 3′ ), 5.33 (br s, 1H, OH), 6.17 (t, J=7.2Hz, 1H, H 1′ ), 7.25-7.35 (m, 5H, H o , H p , H m ), ...
Embodiment 2
[0028] Example 2: Synthesis of 5'-(3-phenylpropionic acid) ester of 5-fluoro-2'-deoxyuridine
[0029] Add 5-fluoro-2'-deoxyuridine (101mg, 0.41mmol), 3-phenylpropionate acetone oxime ester (1.64mmol), 4mL n-hexane / pyridine (1 / 6, volume ratio) into a Erlenmeyer flask with a seal stopper Next, 100 mg of Bacillus subtilis protease was added, placed in a constant temperature shaker at 60° C. and 250 rpm under normal pressure, and the reaction was monitored by TLC. After the reaction, filter, concentrate the filtrate under vacuum, and finally purify by column chromatography to obtain 133 mg of the product, with a yield of 86%, as a white powder, with a purity greater than 99%. The Rf value and NMR data of 5'-(3-phenylpropionic acid) ester of 5-fluoro-2'-deoxyuridine on TLC are as follows: Rf=0.33 (PE / EA=1 / 2); 1 H NMR (DMSO-d 6 )δ: 2.11-2.24 (m, 2H, H 2′ ), 2.68(t, J=7.6Hz, 2H, H 2″ ), 2.87(t, J=7.6Hz, 2H, H 3″ ), 3.93 (br s, 1H, H 3′ ), 4.20-4.25 (m, 3H, H 4′ , H 5′ ), 5.34(b...
Embodiment 3
[0030] Example 3: Synthesis of 3'-(3-phenylpropionic acid) ester of 5-fluoro-2'-deoxyuridine
[0031] 5-fluoro-2'-deoxyuridine (101mg, 0.41mmol), 3-phenylpropanoic acid vinyl ester (8.2mmol), 30mL[C 4 MIm]BF 4 Put it into a Erlenmeyer flask with a sealed stopper, then add 50 mg of immobilized lipase derived from Burkholderia cepacia, place it in a constant temperature shaker at 40°C and 200 rpm under normal pressure, and monitor the reaction by TLC. After the reaction, filter, extract, remove the solvent under vacuum, and finally purify by column chromatography to obtain 139 mg of the product, the yield is 90%, white powder, and the purity is greater than 99%. The Rf value and NMR data of 3'-(3-phenylpropanoic acid) ester of 5-fluoro-2'-deoxyuridine on TLC are as follows: Rf=0.36 (PE / EA=1 / 1); 1 H NMR (DMSO-d 6 )δ: 2.18-2.29 (m, 2H, H 2′ ), 2.69(t, J=7.6Hz, 2H, H 2″ ), 2.88(t, J=7.6Hz, 2H, H 3″ ), 3.63 (br s, 2H, H 5′ ), 3.95 (br s, 1H, H 4′ ), 5.22(t, J=2.4Hz, H 3′ ),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com