Method for preparing 2'-deoxyuridine by chemical-biological enzyme method in combination
A biological enzyme method and deoxyuridine technology, which is applied in the field of chemical-biological enzyme method combined preparation of 2'-deoxyuridine, can solve the problems of inability to obtain a single isomer, high cost, insufficient raw materials, etc., and achieve environmental friendliness, The effect of strong specificity and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Put 7.02g of orthophosphoric acid into the Erlenmeyer flask, add 100mL of acetonitrile, add 5.6mL of n-tributylamine, 10g of 4A molecular sieve (Shanghai Jialin Molecular Sieve Co., Ltd.) 8.5 g of bis(O-p-chlorobenzoyl)-2-deoxy-D-ribose was stirred and reacted for 13 hours under nitrogen protection, then 16.0 mL of n-tributylamine was added, and the reaction was continued for 8 hours to obtain a wine-red transparent liquid, which was removed by filtration. Molecular sieves, and the filtrate was evaporated to dryness to obtain a reddish-brown syrupy substance. After dissolving the syrupy substance in 120mL of tetramethyldipentanone, wash with 100mL of water three times, cool the obtained organic phase to 0°C, add 5.60mL of cyclohexylamine and stir for 0.5h to precipitate a white precipitate, filter it, and wash the filter cake with tetramethyldipentanone Wash with pentanone and acetone, and dry in vacuo to obtain 12.78 g of a white solid with a yield of 94.03% and a puri...
Embodiment 2
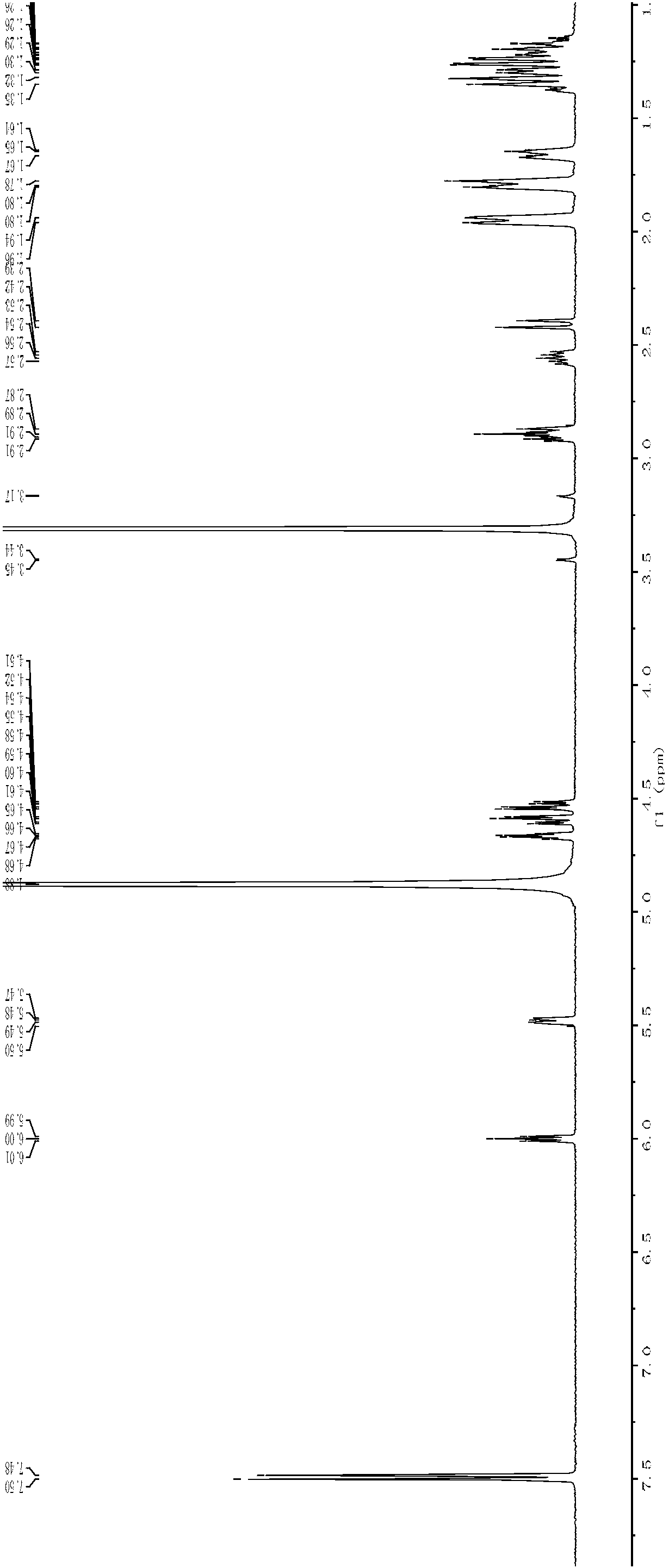
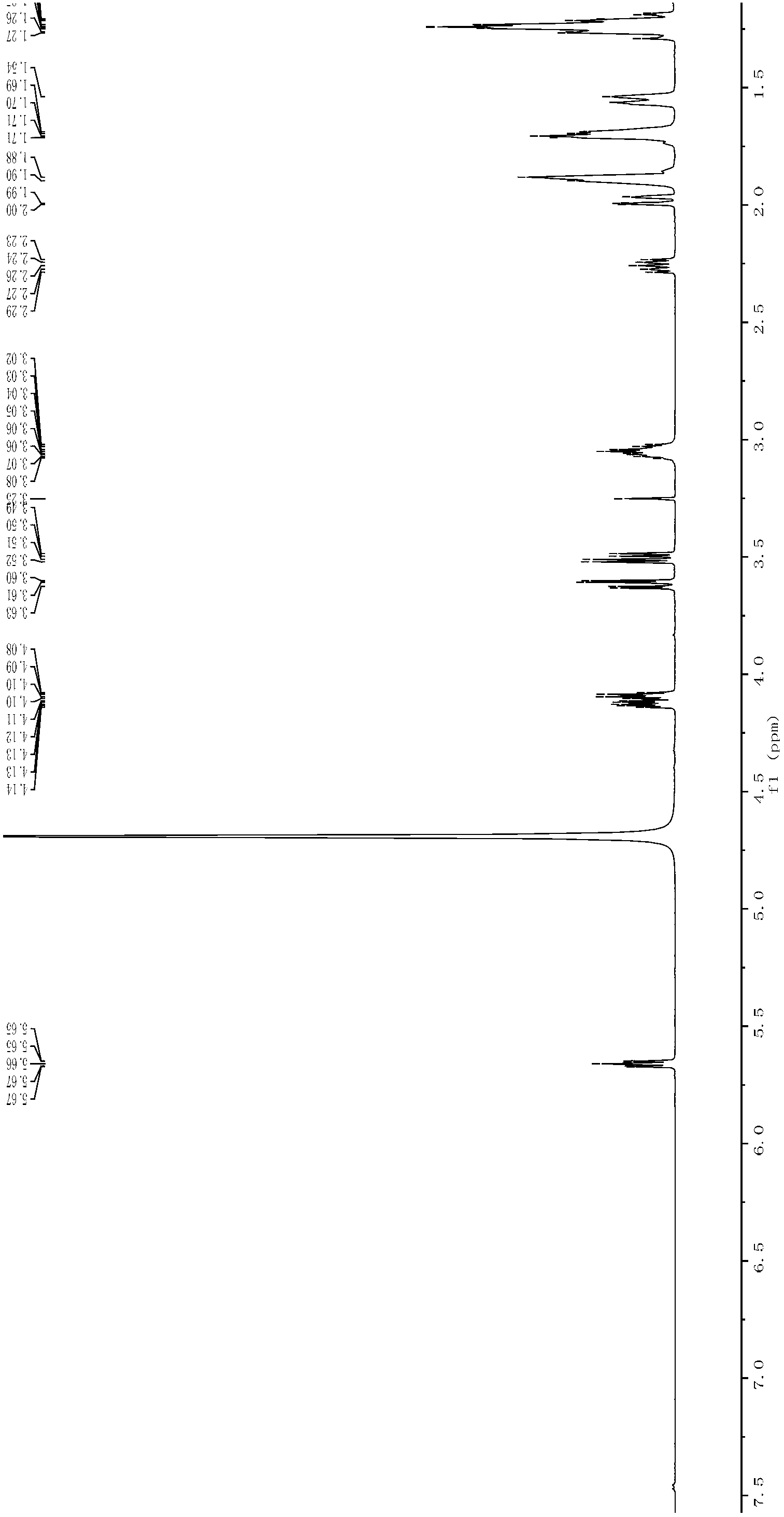
[0042] Dissolve 7.02g of 3,5-O-bis(4-benzoyl chloride)-2-deoxy-α-D-ribose-1-phosphate dicyclohexylamine salt in 120mL of methanol, add 3.0mL of cyclohexylamine, and stir at 40°C After reacting for 24 h, the reaction solution was concentrated, and the final precipitate was washed with ethanol and dried in vacuo to obtain a white solid (3.46 g, 89.4%). IR, 1 H NMR spectrum see image 3 , Figure 4 ,Data are as follows:
[0043] Melting point mp: 166-167°C; IR(KBr): 2939cm -1 , 2853cm -1 , 2238cm -1 , 1630cm -1 ;
[0044] 1 H NMR: 5.67 (1H, dd), 4.14-4.07 (2H, ddd), 3.60 (1H, dd), 3.52 (1H, dd), 3.07 (2H, m), 2.28-2.23 (1H, ddd), 1.99 (1H, ddd), 1.89 (4H, m), 1.71 (4H, m), 1.57 (2H, m), 1.29-1.21 (8H, m), 1.12 (2H, m).
Embodiment 3
[0046] Uracil 0.120g, 2-deoxy-α-D-ribose-1-phosphate 0.432g, Escherichia coli ATCC8379 wet bacterium 2.48g (the strain comes from the Guangdong Provincial Institute of Microbiology, and the freeze-dried tube strain is activated and cultivated on an inclined plane , inoculated into the enzyme production medium, the composition of the enzyme production medium is: peptone 10g / L; yeast extract powder 5g / L; sodium chloride 10g / L, pH 7.0, cultured in a shaker at 36°C and 250r / min for 24h, then centrifuged , collected wet cells and sonicated), dissolved in 50 mL of phosphate buffer solution with pH=7, reacted in a constant temperature water bath at 65°C for 6 hours, diluted the reaction solution by 100 times, and detected it by HPLC.
[0047] Liquid chromatography conditions: column, SB-C 18 column (5 μm, 4.6×250 mm); detection wavelength: 262; mobile phase: methanol: water = 9:1; flow rate: 0.8 mL / min; injection volume: 20 μL.
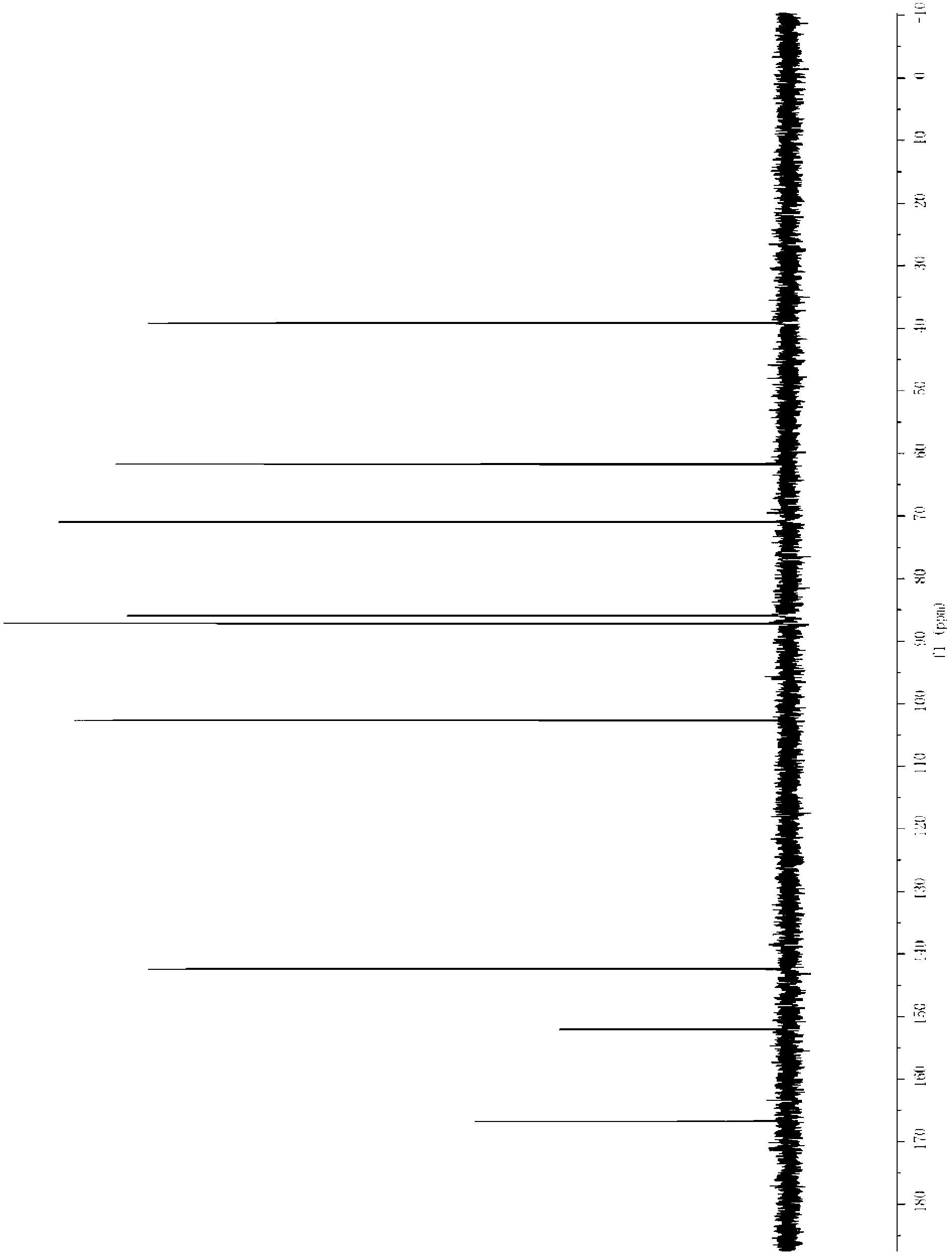
[0048] The isolated 2′-deoxyuridine 13 C NMR, 1 H ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com