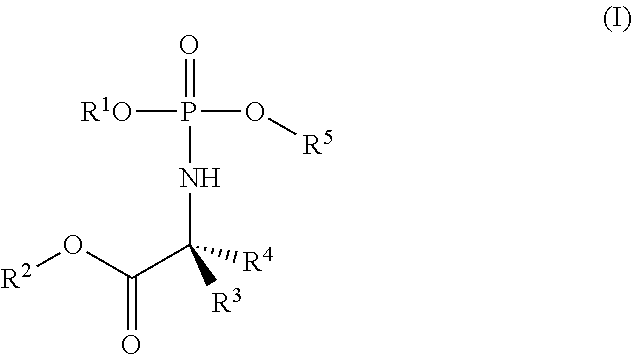
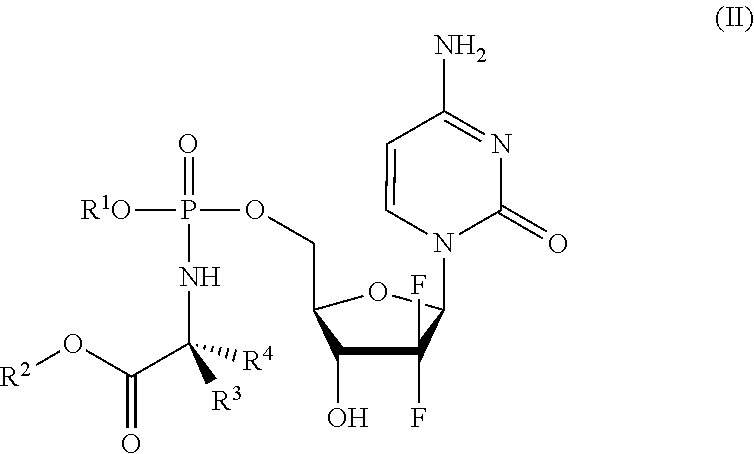
Formulations of phosphoramidate derivatives of nucleoside drugs
a technology of phosphoramidate and derivatives, which is applied in the direction of pharmaceutical delivery mechanism, drug composition, organic active ingredients, etc., can solve the problems of limited clinically acceptable methods for delivering compounds at sufficiently high dosages for effective treatment, and the protide is often extremely lipophilic and thus poorly water soluble, so as to achieve the effect of reducing the risk of precipitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
y of NUC-3373
[0238]The solubility of NUC-3373 (mixture of diastereoisomers) in a range of solvents is shown in Table 1.
TABLE 1Solubility of NUC-3373 in a range ofpharmaceutically relevant solventsNUC-3373Solvent(mg / mL)Ethanol778Propylene glycol449PEG 400422NMP705DMSO948DMA950Water
As can readily be seen, the solubility of NUC-3373 in water is extremely low. Of the solvents tested, the polar aprotic solvents and particularly DMSO and DMA offered the best solubilities.
example 2
nt of an Aqueous Formulation of NUC-3373
[0239]The successful development of the Diluent Solution to enable preparation of the NUC-1031 aqueous formulation prompted its development for an aqueous formulation of NUC-3373. An aqueous NUC-3373 formulation was developed by adding 6.7 ml of a 250 mg / ml solution of NUC-3373 in 80% DMA:20% 0.9% saline to 10 ml diluent solution to generate a 100 mg / ml NUC-3373 surfactant solution (see Table 4), prior to subsequent dilution into an infusion bag.
The clinical dose for NUC-3373 has yet to be established, but the estimated maximum dose may be up to 3,000 mg, which set the upper limit for the formulation development studies. Table 2 shows the volume of 100 mg / ml NUC-3373 surfactant solution that is required to be added to a 250 ml infusion bag for a variety of doses, and the resulting composition of the aqueous infusion solution.
TABLE 2Composition of saline infusion solution across a variety of doses of NUC-3373.NUC-3373 Dose (mg)1,000 mg2,000 mg3...
example 3
ive Description of a Formulation Methodology
[0241]A formulation methodology (see WO2015 / 198059 (PCT / GB2015 / 051858)) has been developed for the intravenous administration of protides. This methodology has been shown in clinical trials to be effective for NUC-1031 which has broadly the same solubility profile as NUC-3373 and NUC-7738. That methodology is as follows:
A 250 mg / mL solution of the protide (the S-epimer, the R epimer or a mixture thereof) is formed in an 80:20 (by volume) mixture of DMA and 0.9% saline. This stock solution formulation is typically sufficiently stable for long term storage and transport of protides.
This stock solution formulation can be administered to patients intravenously via a CVAD (e.g. a Hickman line, PICC line). The intravenous administration apparatus will typically be flushed with an 80:20 (by volume) mixture of DMA and 0.9% saline both before and after administration of the formulation comprising the protide. This helps mitigate the risk of any pot...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com