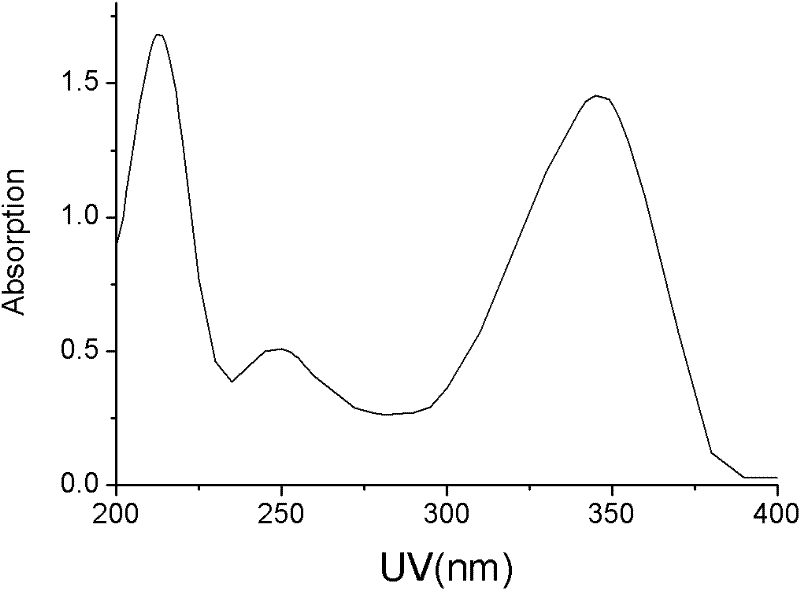
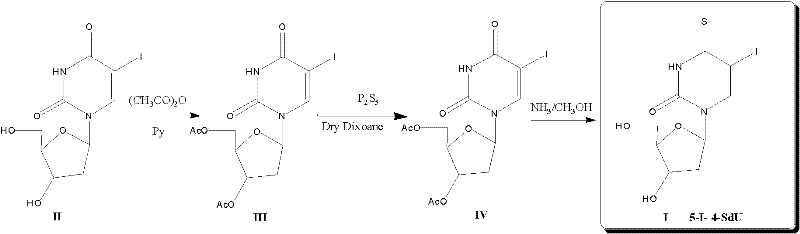
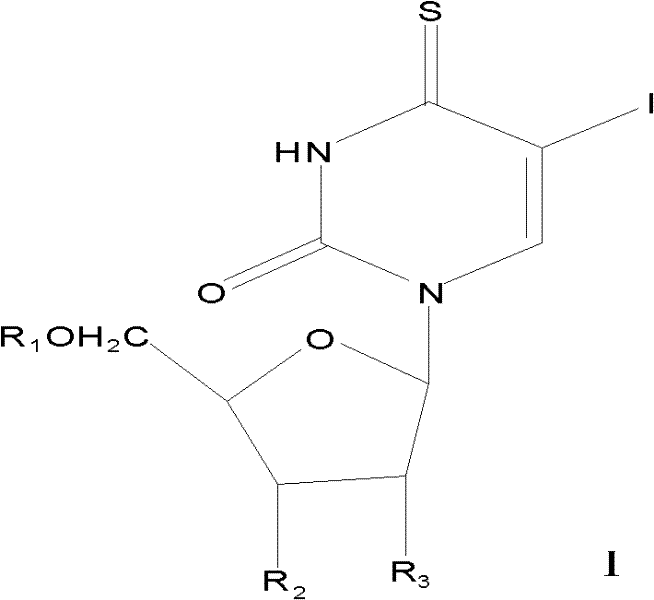
5-iodine-4-sulfur-2'-deoxyuridine, and derivatives and synthetic method thereof
A technology of deoxyuridine and its synthesis method, which is applied in the direction of preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problem of inability to selectively act on cancer cells, etc., and achieve the improvement of industrial application prospects, wide application prospects, and purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: A kind of synthetic method of the synthetic method of 2'-deoxy-5-iodo-4-thiouridine comprises the following steps:
[0056] (1) Synthesis of 3', 5'-O-dioxoacetyl-5-iodo-2'-deoxyuridine [III] by acetylation, the reaction formula is as follows:
[0057]
[0058] In a 100ml three-strength flask, add 1.00g (2.82mmol) 5-iodo-2'-deoxyuridine [II], 15ml anhydrous pyridine, and after it is fully dissolved, add 3.0ml (32mmol) acetic anhydride, in ice React under bath conditions for 16h, remove the solvent under reduced pressure, then add 10ml each of dichloromethane and benzene, remove the solvent under reduced pressure, and then add 20ml of dichloromethane to remove the solvent under reduced pressure. The crude product was dissolved in 250ml of dichloromethane, then added with 80ml of saturated sodium bicarbonate, and extracted three times with dichloromethane. The organic layer was dried over excess anhydrous sodium sulfate, filtered, and the solvent was remo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com