T cell activation
a technology activation, which is applied in the field of t cell activation, can solve the problems of contamination problems, undesirable infecting individuals with antigenic substances, and activation of t cell specific for antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
Materials and Methods
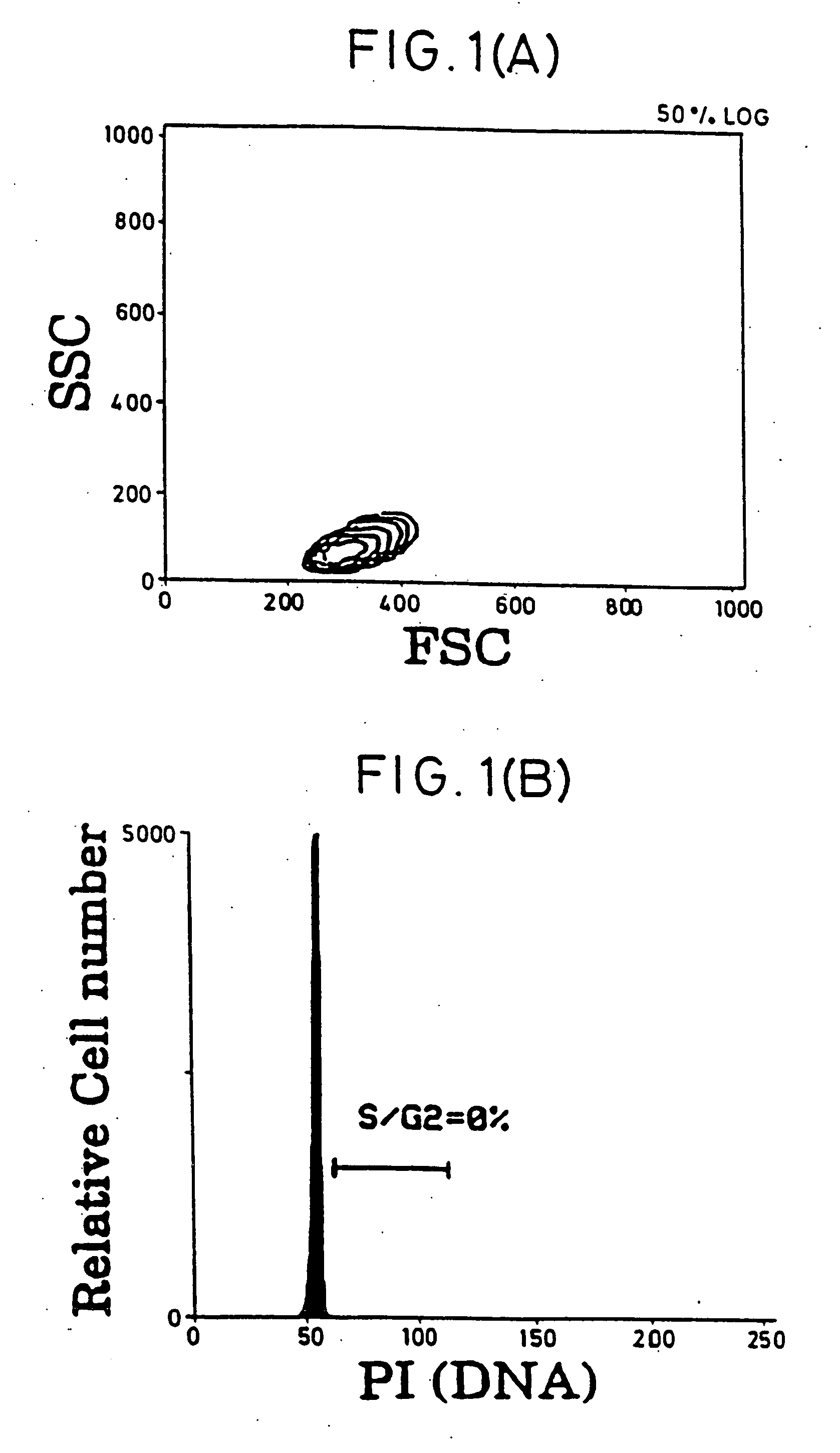
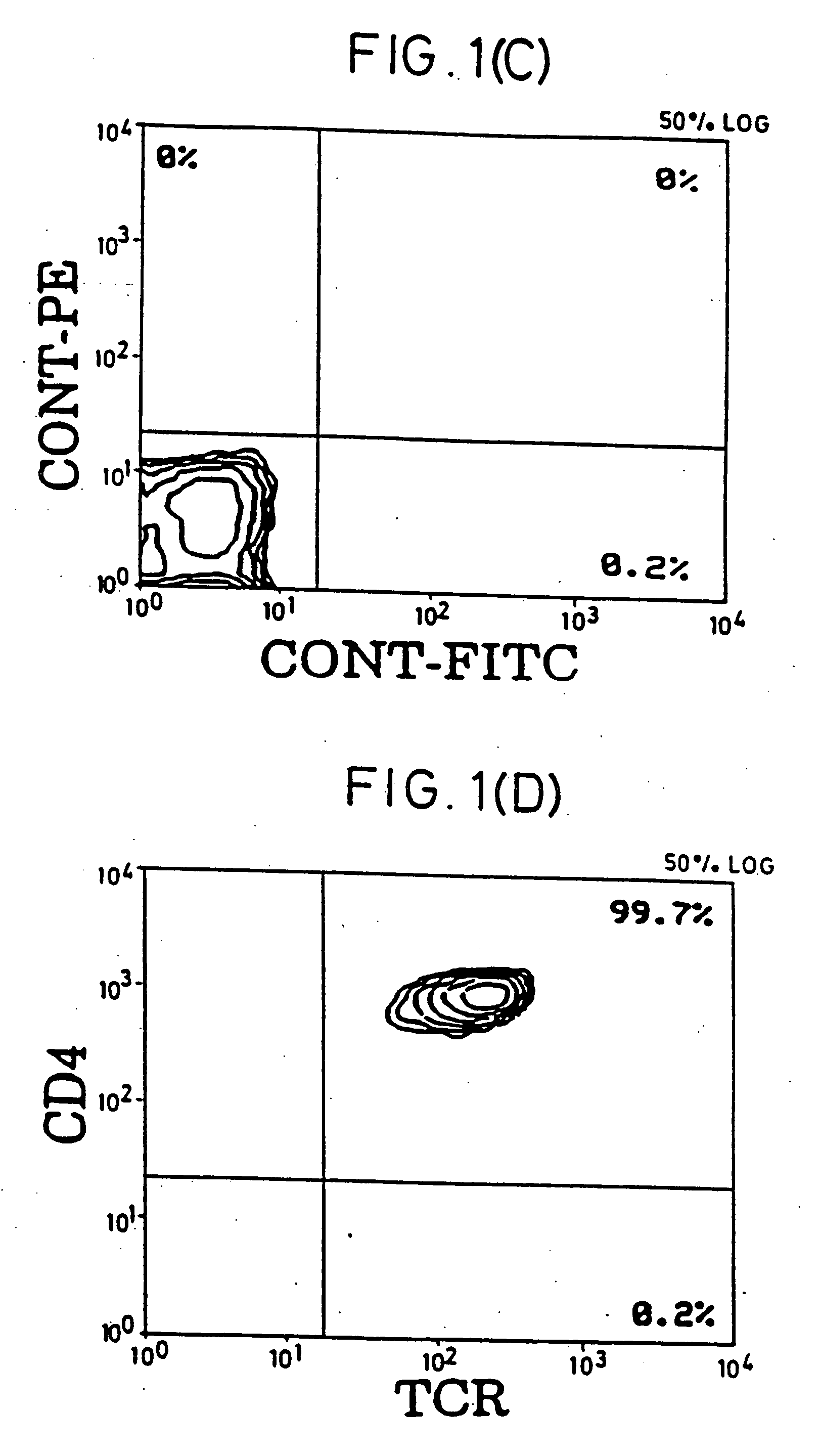
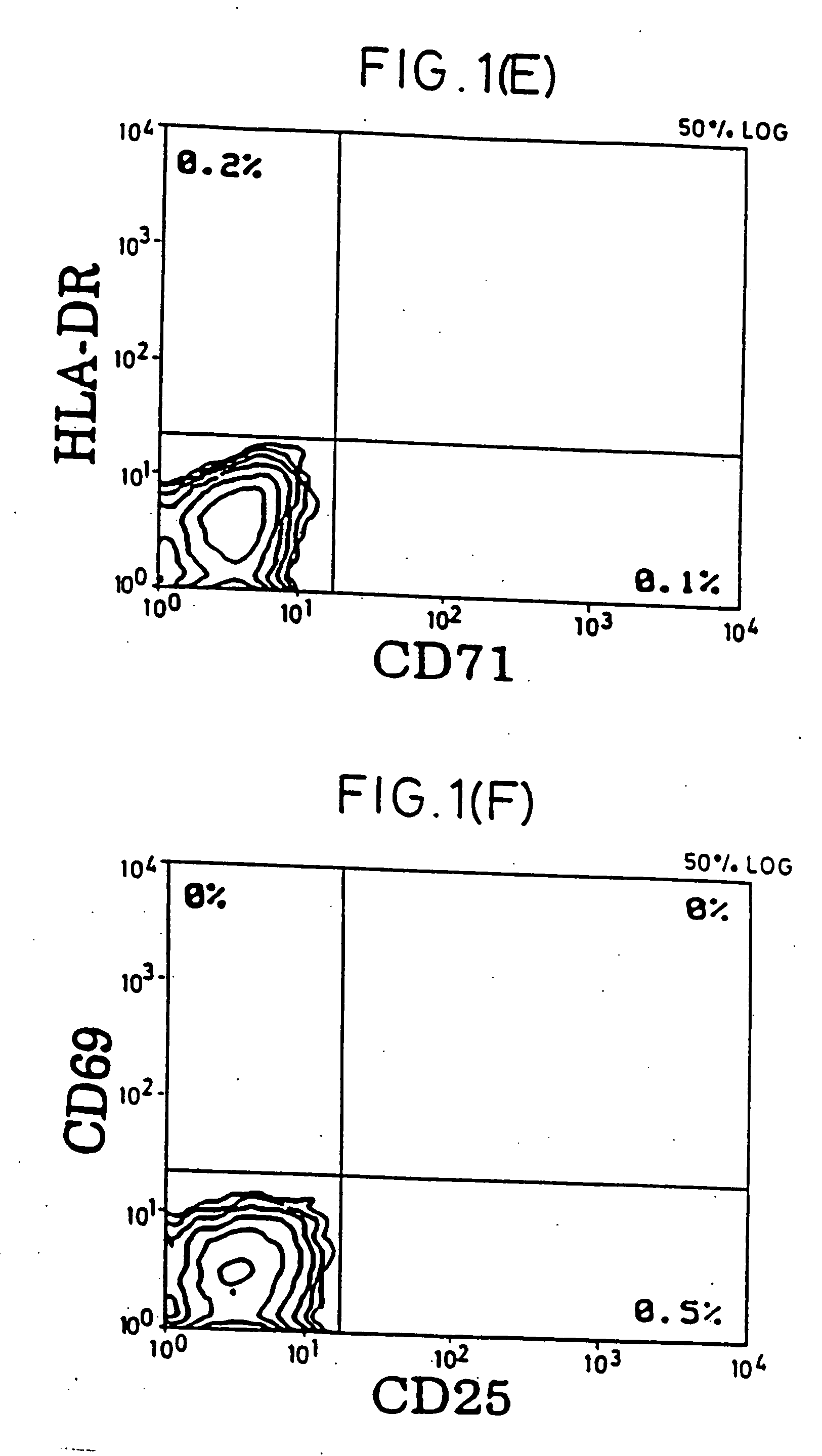
[0050] Purification of Resting T Cells. After Ficoll-Hypaque (pharmacia) separation of PBMC from buffy coats of healthy donors, most macrophages were removed by plastic adherence. To obtain a pure resting CD4+ T cell population, cells were incubated with a cocktail of mAbs against HLA-DR (L-243; American Type Culture Collection [ATCC], Rockville, Md.), CD19 (4GT), CD16 (B73.1), CD56 (MY31), CD57 (HNK-1, ATCC), CD8 (OKT8, ATCC), CD11b (OKM-1, ATCC), CD14 (MO-P9), TCR-c / δ (B1, a gift of G. De Libero, ZLF Basel, Switzerland), CD25 (2A3), CD69 (L78), and CD71 (L01.1). After 30-min incubation on ice, cells were washed twice and incubated with magnetic beads (Dynabeads; Dynal, Oslo, Norway) conjugated with goat anti-mouse IgG and rat anti-mouse IgM, at a 1:4 target / bead ratio. After 30-min incubation, bead-bound cells were removed using rare earth magnet (Advanced Magnetics, Inc., Cambridge, Mass.). Remaining cells were further purified with four more incubations wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com