Treatment of diseases with alpha-7nACh receptor full agonists
An agonist, disease technology, applied in metabolic diseases, skin diseases, sensory diseases, etc., can solve problems such as limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
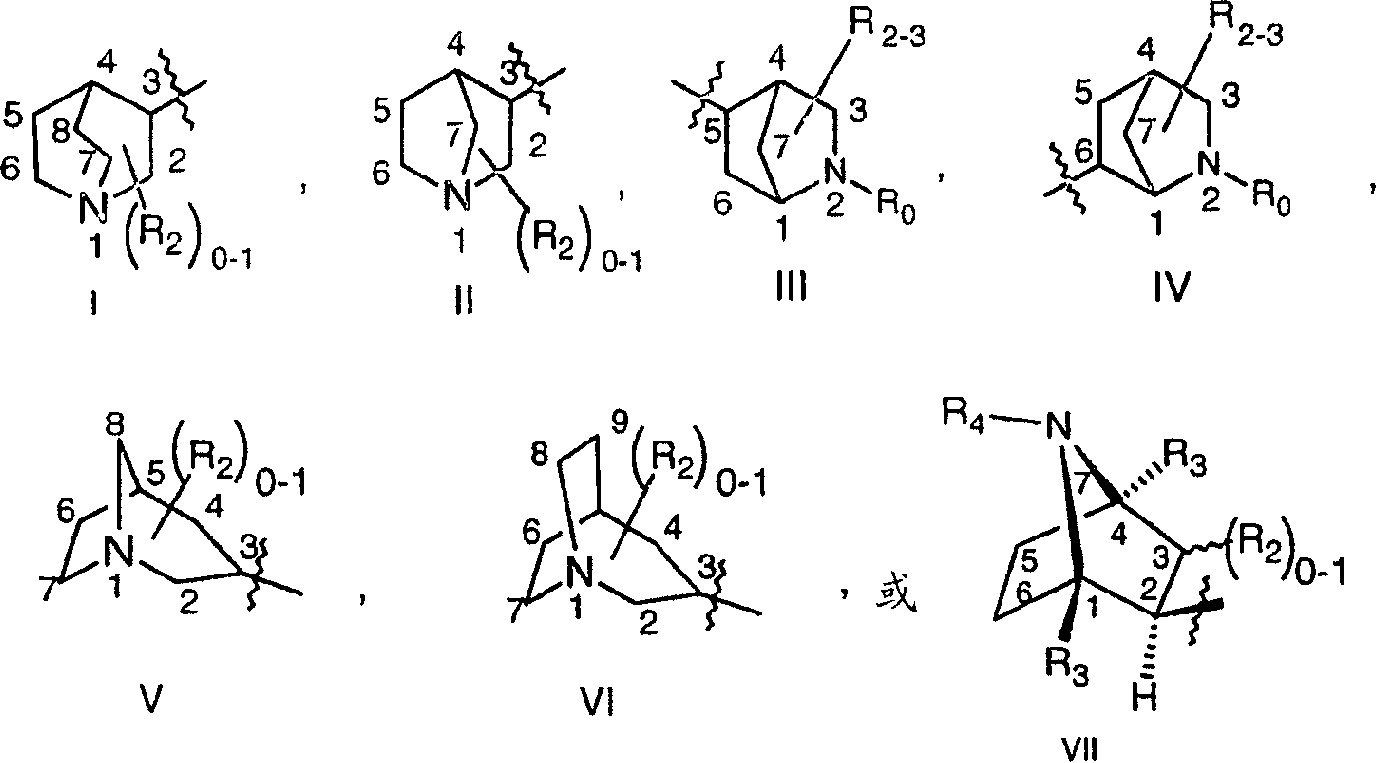
Image
Examples
Embodiment
[0785] Embodiment (H): N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-4-bromo-1H-pyrazole-1-carboxamide hydrochloride:
[0786]
[0787] A solution of 4-bromopyrazole (0.52 g, 3.5 mmol) in 30 mL of EtOAc was added to a solution of excess phosgene (10 mL, 20% in toluene) in EtOAc. After the addition was complete, the solution was refluxed for 1 hour, cooled and concentrated in vacuo. EtOAc was added and the mixture was concentrated again. The residue was treated with 20 mL of THF, (R)-(+)-3-aminoquinucidine dihydrochloride (0.71 g, 3.5 mmol) and excess TEA (5.0 mL, 68.1 mmol). After 60 hours 1N NaOH solution was added. Mixture with CHCl 3 Extracted, dried (MgSO 4 ), filtered and concentrated. The residue was subjected to flash chromatography (Biotage 40S, 90:9:1 CHCl 3 / MeOH / NH 4 OH) purification. After preparing Example 1(H), it was recrystallized from MeOH / EtOAc to afford 289 mg (25%) of a white solid. to C 11 h 15 BrN 4 HRMS (FAB) calcd for O+H 299.0508, found 299.051...
Embodiment 2
[0788] Example 2(H) : N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-4-iodo-1H-pyrazole-1-carboxamide hydrochloride:
[0789]
[0790] Phenyl chloroformate (0.75 mL, 6.0 mmol) was added dropwise to a solution of 4-iodopyrazole (1.05 g, 5.4 mmol) and TEA (0.9 mL, 6.5 mmol) in 15 mL of CH 2 Cl 2 in solution. The reaction was stirred at room temperature. After 60 hours water was added. Mixture with CH 2 Cl 2 Extracted, dried (MgSO 4 ), filtered and concentrated. After addition of hexane the solvent was removed in vacuo. A white solid formed on standing, yielding 1.6 g (95%) of phenyl 4-iodo-1H-pyrazole-1-carboxylate. MS (EI) m / z 315.1 (M + ).
[0791] 4-Iodo-1H-pyrazole-1-carboxylic acid phenyl ester (1.6g, 5.2mmol) and (R)-(+)-3-aminoquinuclidine dihydrochloride (1.0g, 5.2mmol) Suspended in 10 mL DMF. DIEA (2.7 mL, 15.5 mmol) was added dropwise. After 36 hours the solvent was removed and the residue was taken into 1N NaOH and CHCl 3 middle. CHCl for aqueous layer 3 Ex...
Embodiment 3
[0792] Embodiment 3 (H): N-[(3R)-1-azabicyclo[2.2.2]oct-3-yl]-4-(2-chlorophenyl)-1H-pyrazole-1-carboxamide hydrochloride:
[0793]
[0794] Hydrazine hydrate (0.55 mL, 11.3 mmol) was added to a solution of 2-chlorophenylpropanedial dissolved in 20 mL of EtOH. The mixture was heated to reflux for 3 minutes, then stirred at room temperature overnight. The solvent was removed in vacuo to give 4-(2-chlorophenyl)-1H-pyrazole as a yellow solid. MS (EI) m / z 177.0 (M - ).
[0795] Dissolve 4-nitrophenyl chloroformate (2.3 g, 11.5 mmol) and 4-(2-chlorophenyl)-1H-pyrazole (2.0 g, 11.0 mmol) in 30 mL CH 2 Cl 2 , cooled to 0°C. TEA (1.7 mL, 12.0 mmol) was added and the reaction was allowed to warm to room temperature. After 30 minutes additional 4-nitrophenyl chloroformate (0.25 g) and TEA were added. Water was added after 1 hour. Mixture with CH 2 Cl 2 Extracted, dried (MgSO 4 ), filtered and concentrated to give a solid. The solid was triturated with hexane, filtered an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com