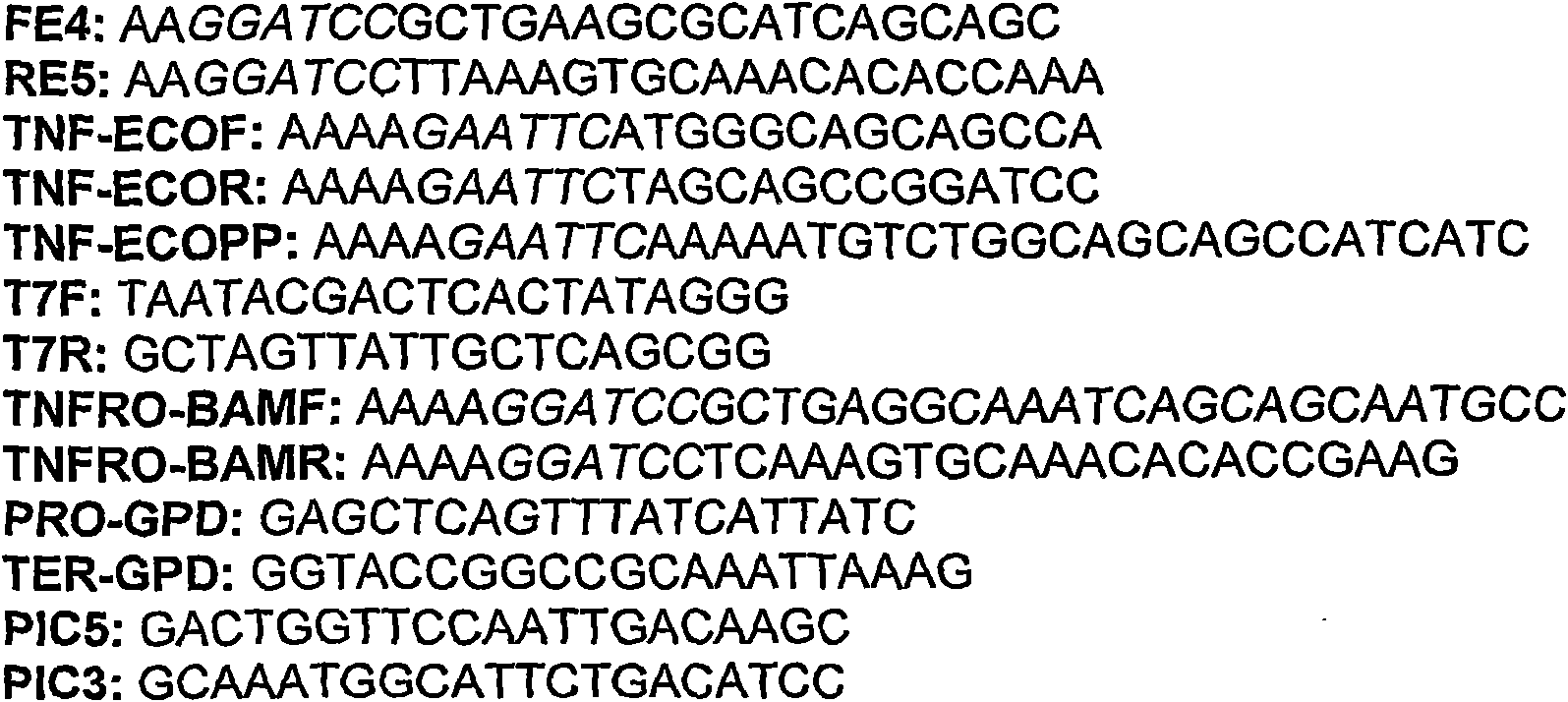Orally administrable immunostimulant product for aquaculture
An immunostimulation and aquaculture technology, applied in the field of orally administered immunostimulation products for aquaculture, can solve the problems of strict dosage control, inactivation, and prevention of intestinal absorption of active antigens, etc., to prevent fish Effects of disease, avoiding side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Expression of gilthead seabream TNFα in E. coli
[0049] Clone sbTNFα into pET15b
[0050] Use LPS-stimulated head kidney cDNA as a template in PCR to use primers FE4 and RE5 ( figure 1 ) Amplify sbTNFα. Both primers contained BamHI restriction sites for subsequent cloning of PCR products in the same site of plasmid pET15b (Novagen). It contains cDNA template, 50μM each dNTP, 0.2mM primer, MgCl 2 1X buffer PLUS and 1 unit of Eco Taq PLUS DNA polymerase (Ecogen) for amplification. The cyclic reaction was carried out in the Eppendorf Mastercycler Gradient, including 1 cycle of 95°C for 2 minutes, 25 cycles of 95°C for 45 seconds, 60°C for 45 seconds, and 72°C for 30 seconds, and a subsequent cycle of 72°C for 10 minutes. The PCR product was purified using QIAquick PCR Purification Kit (Qiagen), and 1 unit of T4 DNA ligase (New England BioLabs, Inc.) was used at room temperature to insert a fragment (insert): plasmid = 3:1 ratio with plasmid pGEM-T Easy (Promega) ...
Embodiment 2
[0053] Example 2: Expression of gilthead seabream sbTNFα in Saccharomyces cerevisiae
[0054] Will His 6 -sb TNFα cloned into p424-GPD
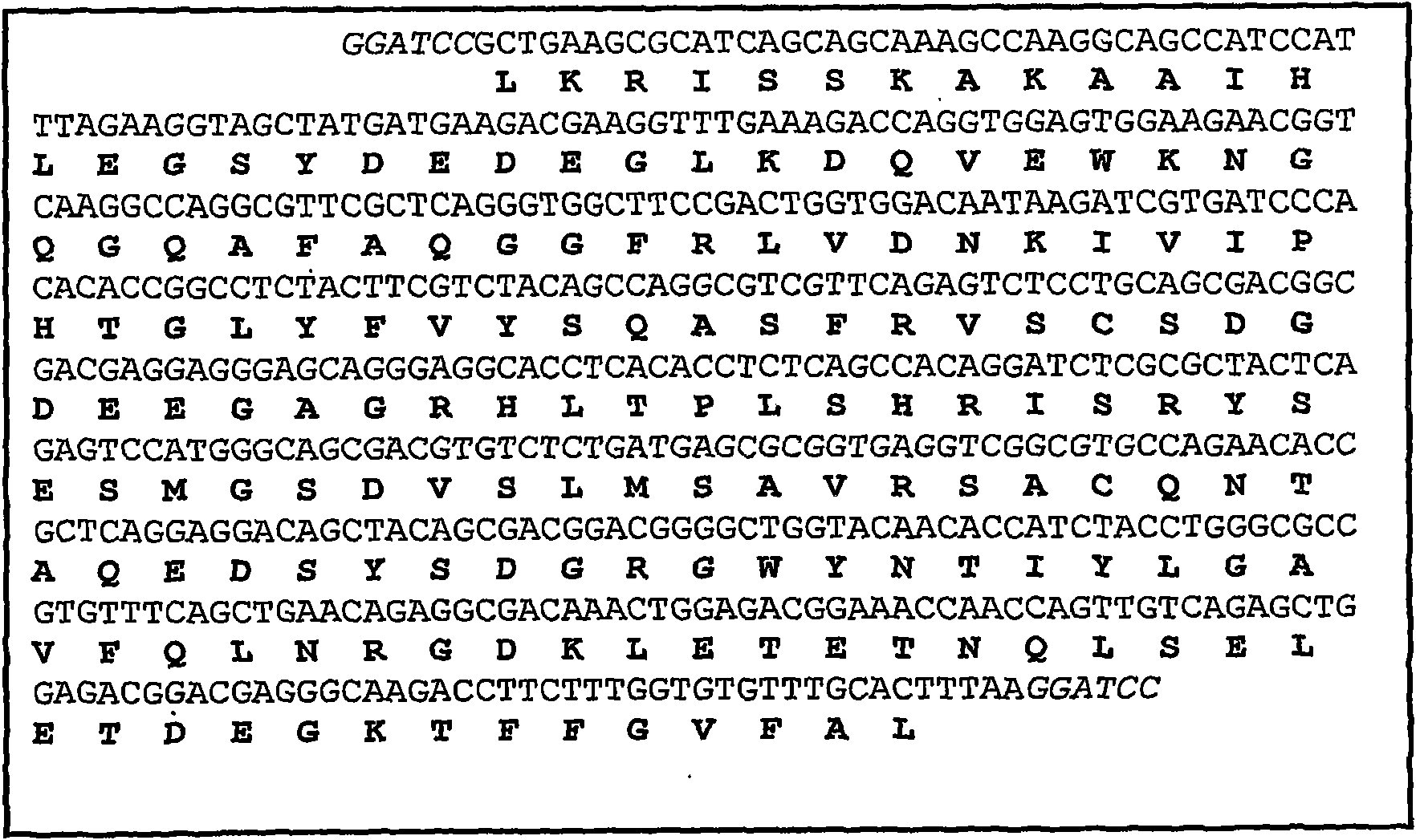
[0055] Use plasmid pET15b containing sbTNFα as a template in PCR to use primers TNF-ECOF and TNF-ECOR ( figure 1 ) Amplification includes 613pb His 6 -Fragments of sbTNFα. Both primers contain EcoRI restriction sites, which are used to clone the PCR product in the same site of plasmid p424-GPD (ATCC 87357). It contains 5ng template, 2.5mM MgCl 2 , Each dNTP 50μM, 0.2mM primers, 1X High Speec additives, 1X buffer PLUS and 2 units Eco Taq PLUS DNA polymerase (Ecogen) sample for amplification. Carry out cyclic reactions in Smart Cycler II (Cepheid), including 1 cycle at 95°C for 5 minutes, 30 cycles at 95°C for 30 seconds, 62°C for 45 seconds, and 72°C for 2 minutes, followed by 1 cycle at 72°C for 10 minutes cycle. The PCR products were separated by electrophoresis in a 0.8% agarose (Pronadisa) gel containing 0.5 μg / ml ethidium bromide, and purif...
Embodiment 3
[0059] Example 3: Expression of gilthead seabream sbTNF in Pichia pastoris
[0060] Will His 6 -sb TNFα cloned into pPICZA
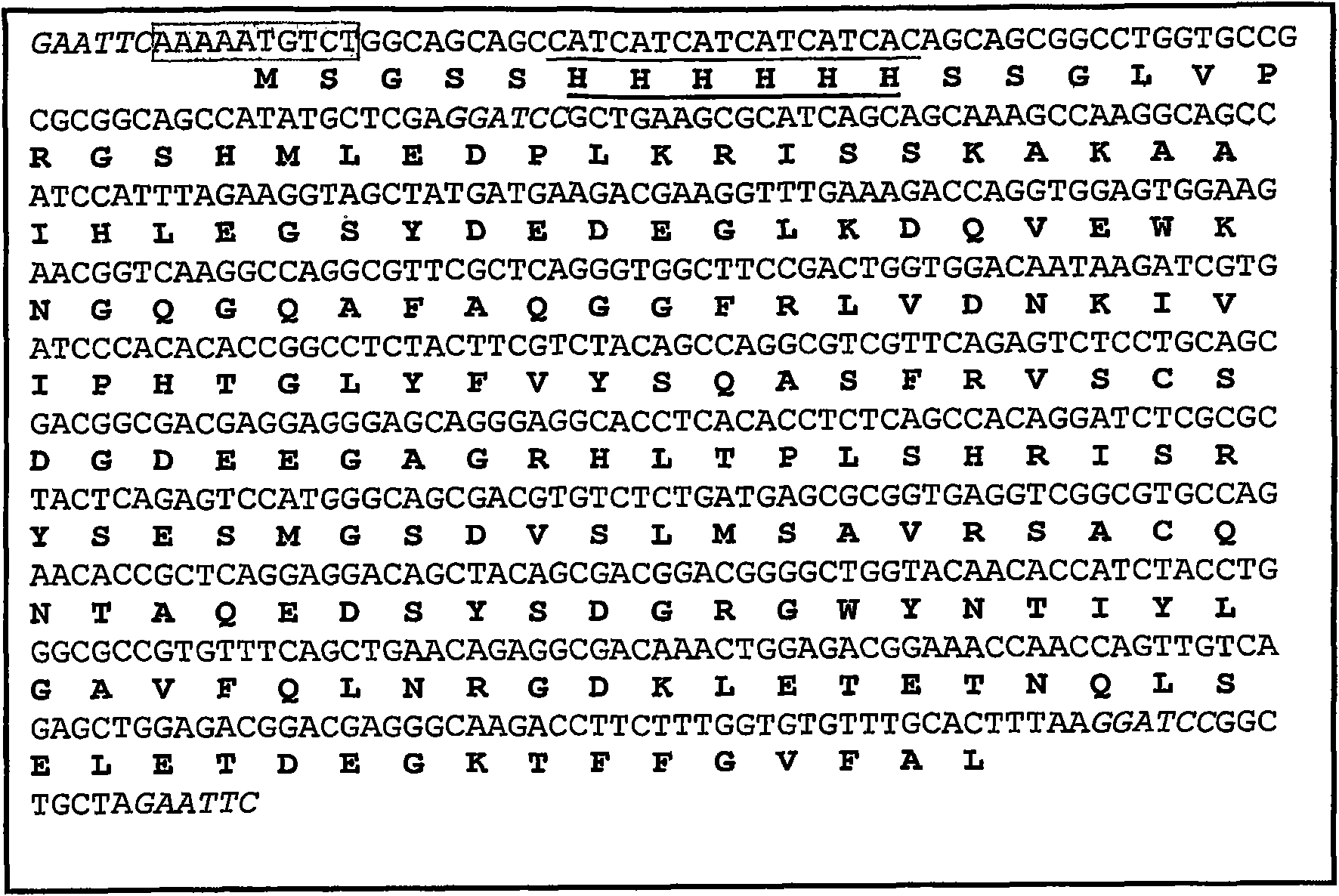
[0061] Use plasmid pET15b containing sbTNFα as a template in PCR to use primers TNF-ECOPP and TNF-ECOR ( figure 1 ) Amplification includes 618pb His 6 -Fragments of sbTNFα. Both primers contain EcoRI restriction sites, which are used to clone the PCR product in the same site of plasmid pPICZA (Invitrogen). It contains 5ng template, 2.5mM MgCl 2 , Each dNTP 50μm, 0.2mM primers, 1X High Speec additives, 1X buffer PLUS and 2 units of Eco Taq PLUS DNA polymerase (Ecogen) for amplification. Carry out cyclic reactions in Smart Cycler II (Cepheid), including 1 cycle at 95°C for 5 minutes, 30 cycles at 95°C for 30 seconds, 55°C for 45 seconds and 72°C for 2 minutes, and then 1 cycle at 72°C for 10 minutes cycle. The PCR products were separated by electrophoresis in a 0.8% agarose (Pronadisa) gel containing 0.5 μg / ml ethidium bromide, and purified using QIAquick Ge...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com