Construction method of mycobacterium tuberculosis fusion protein Mtb 10.4-Hsp16.3, expression method thereof, purification method thereof and application thereof
A technology of mtb10.4-hsp16.3 and Mycobacterium tuberculosis, applied in the field of fusion proteins, can solve the problems of unstable protection and achieve the effects of prolonging the protection effect, improving the protection effect and strengthening the immune effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
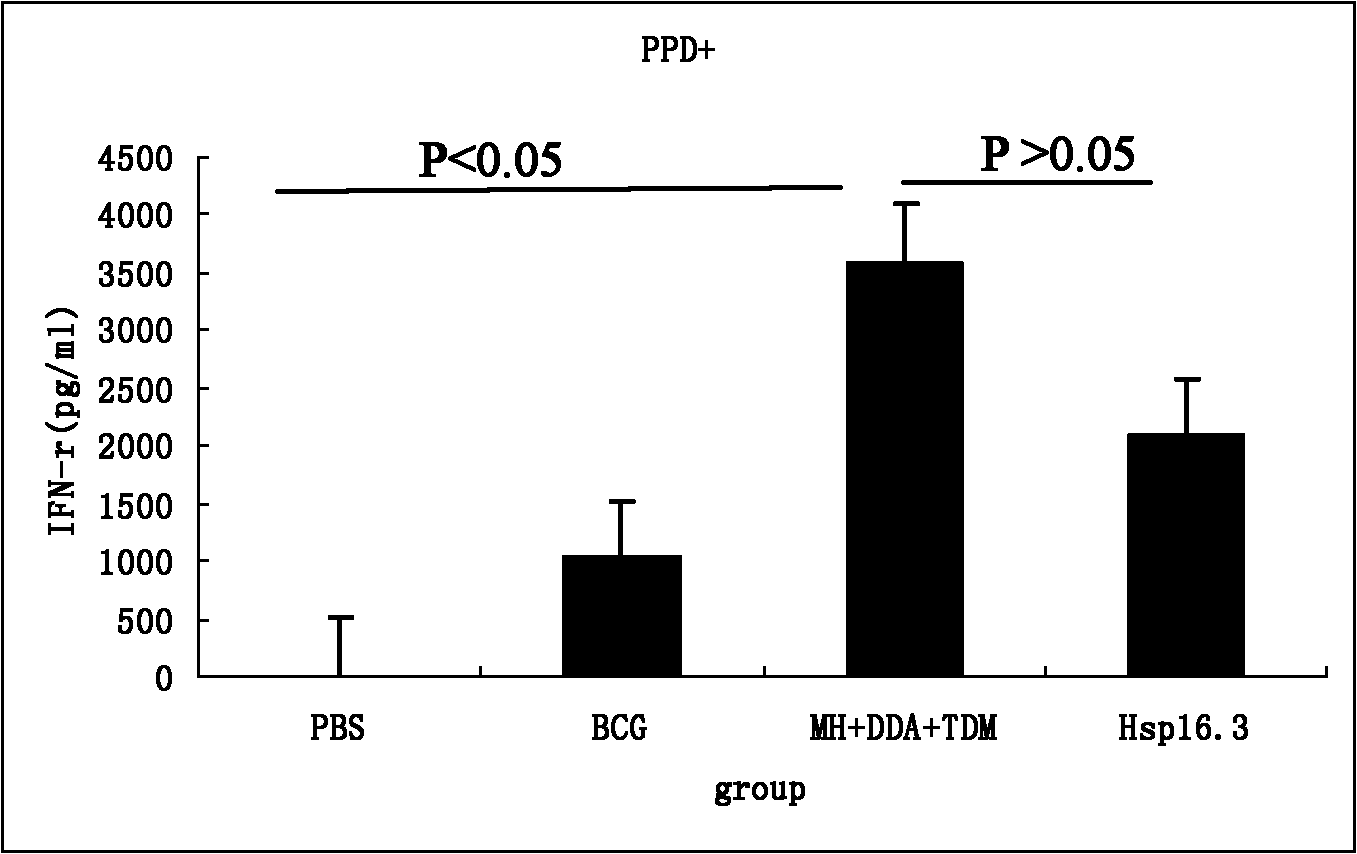
Image
Examples
Embodiment Construction
[0040] Preferred embodiments of the present invention are described below, and it should be understood that the preferred embodiments described here are only used to illustrate and explain the present invention, and are not intended to limit the present invention.
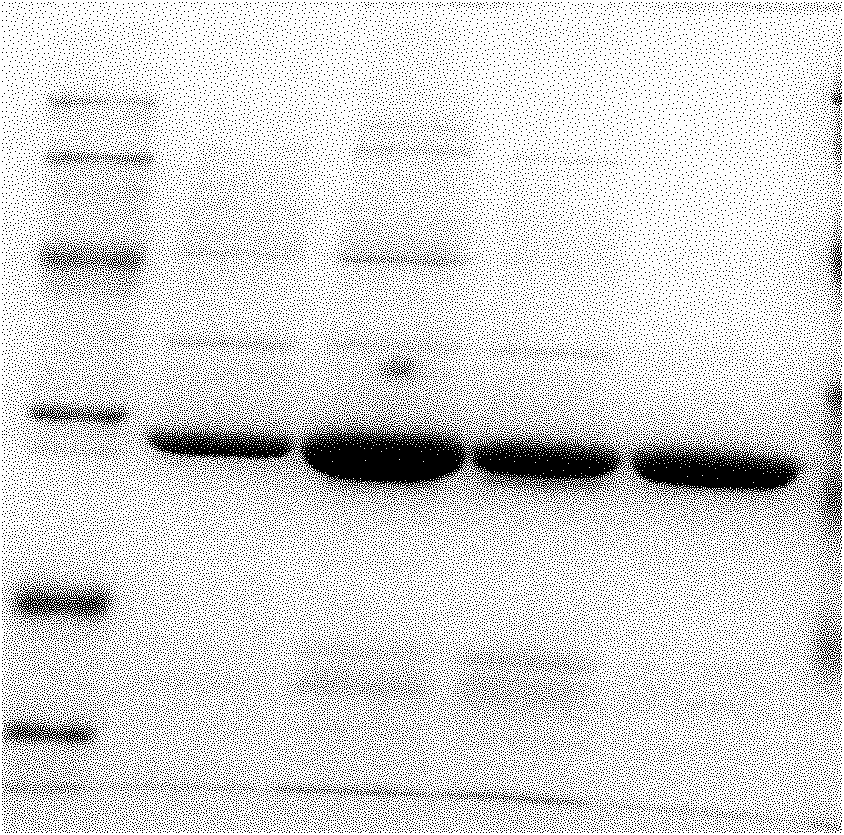
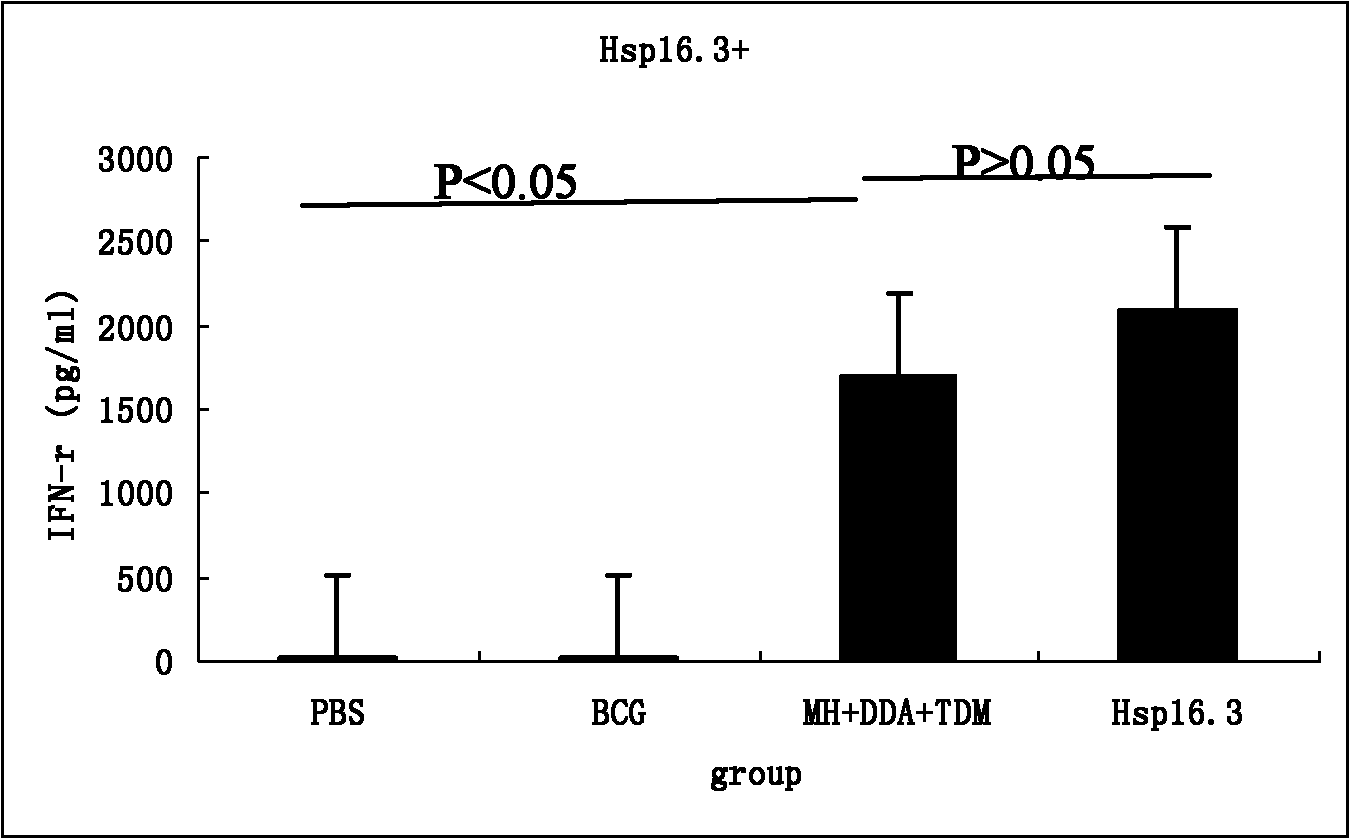
[0041] 1. Construction, expression and purification of Mycobacterium tuberculosis fusion protein Mtb10.4-Hsp16.3
[0042] First, the Mtb10.4 and Hsp16.3 genes were amplified by PCR and inserted into the multiple cloning site of the cloning vector in sequence to construct a recombinant vector; then the above recombinant vector was used to express the fusion protein Mtb10.4-Hsp16 in Escherichia coli. 3; Finally, purify according to the molecular weight, charge and affinity of the fusion protein Mtb10.4-Hsp16.3, and obtain the fusion protein Mtb10.4-Hsp16.3 through purification. The specific steps are as follows:
[0043] 1. Construction of fusion protein Mtb10.4-Hsp16.3(MH):
[0044] (1) According to Mtb10.4 (Rv0288...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com