Immune effector cells pre-infected with oncolytic virus
a technology of oncolytic virus and effector cells, which is applied in the direction of antibody medical ingredients, dsdna viruses, drug compositions, etc., can solve the problems of individual death, tumor enlargement and vital area occupied space, and affect the control mechanism, so as to improve the cytotoxic effect of effector cells, minimal viral infection, and enhanced tumor biodistribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
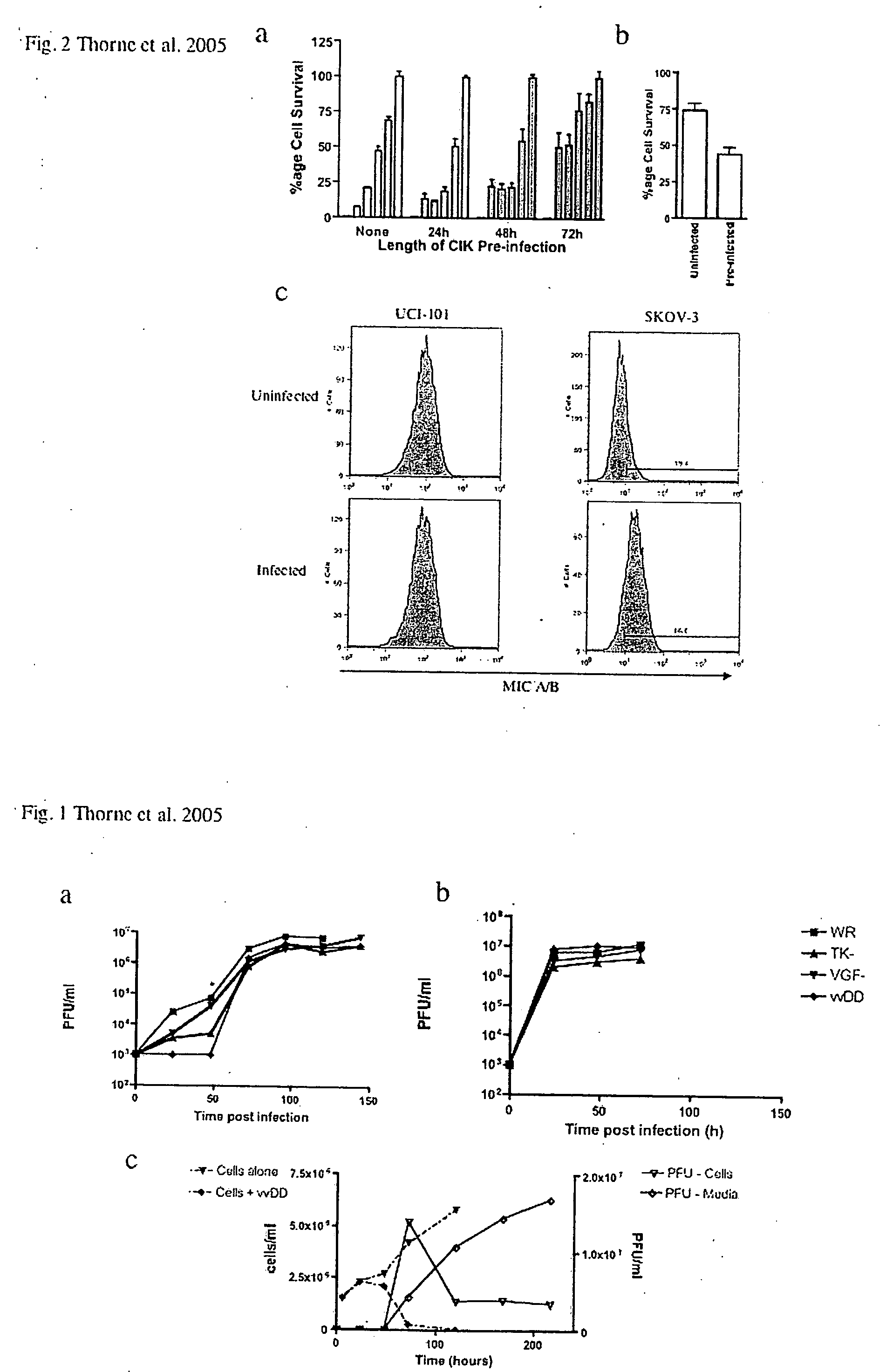
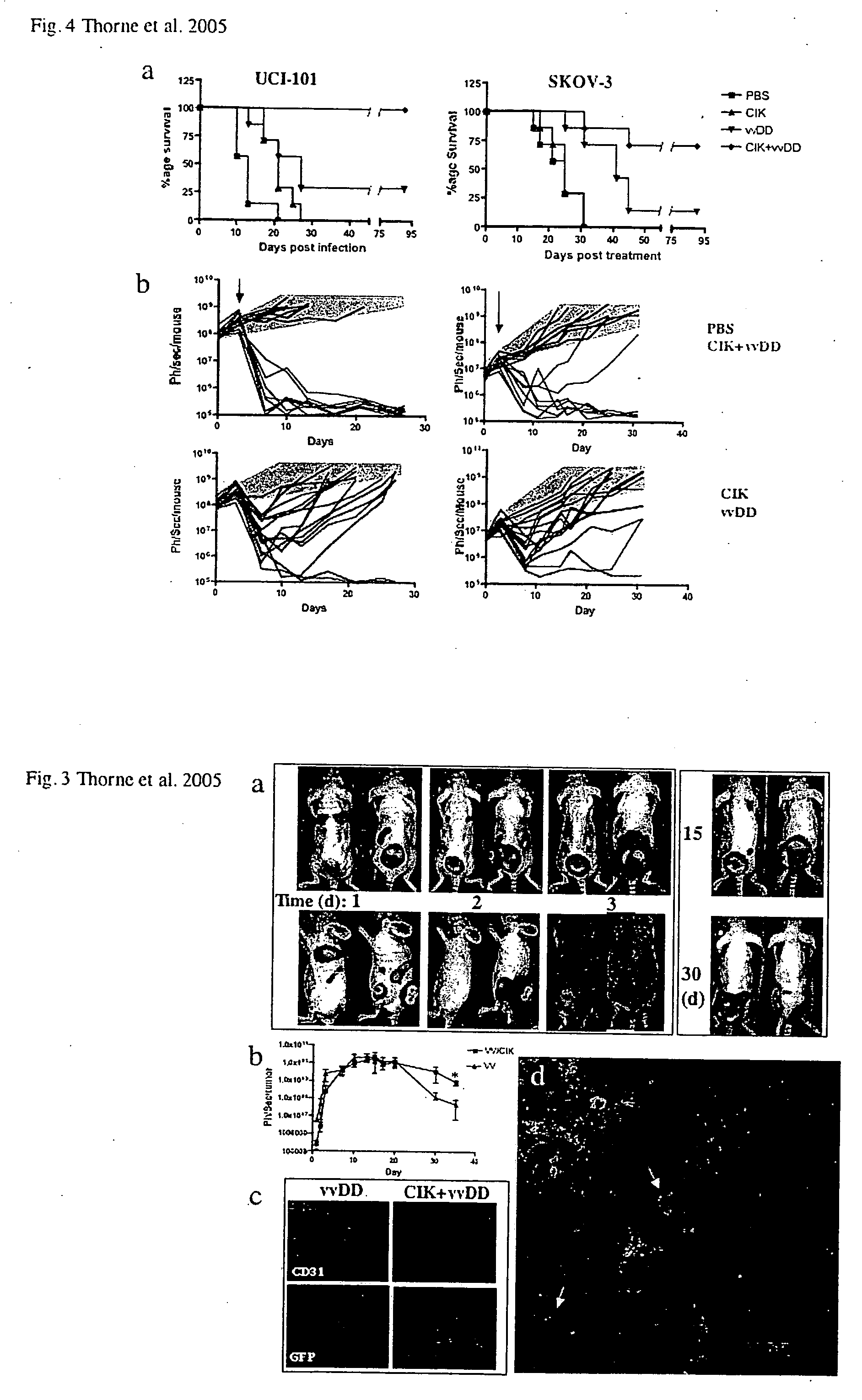
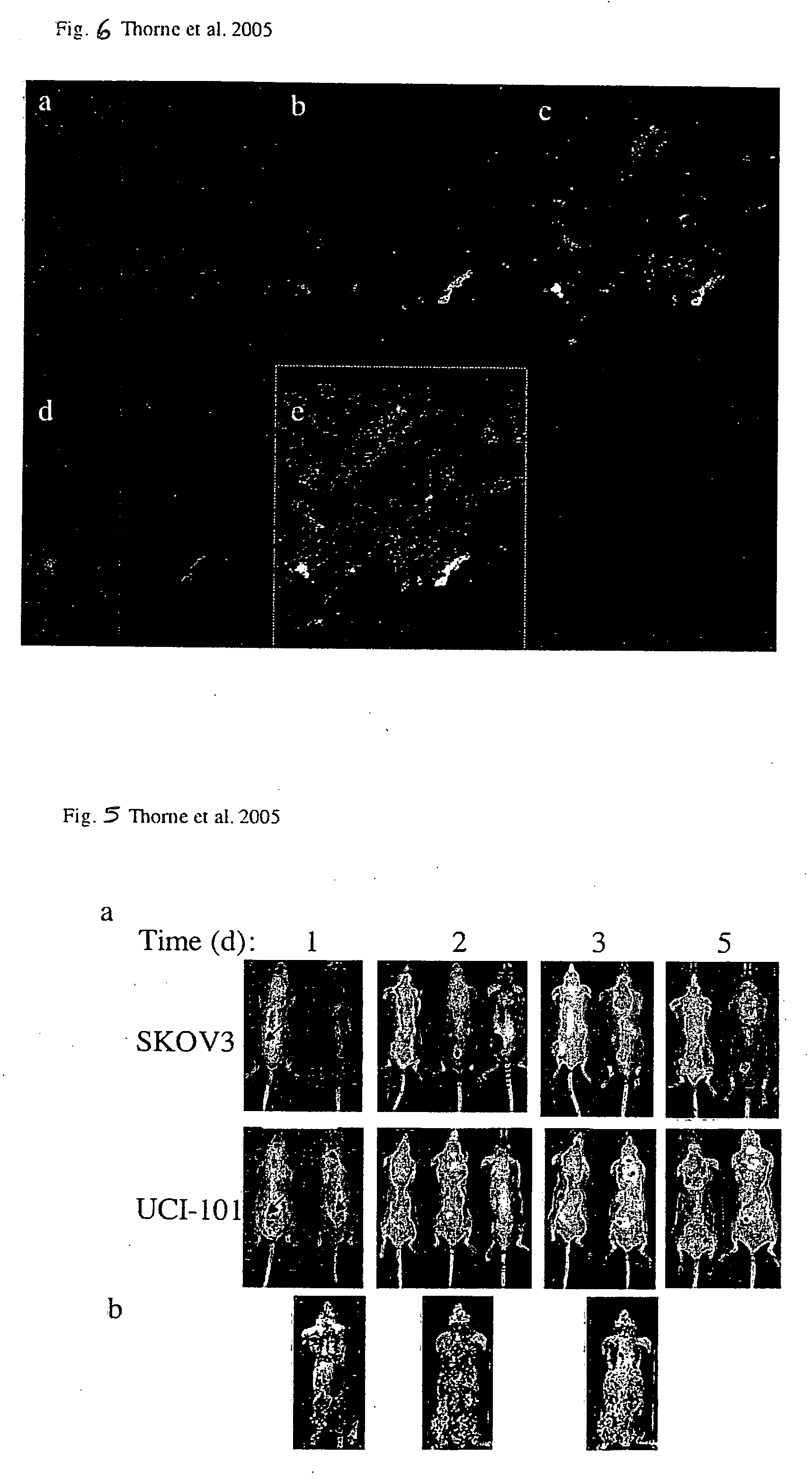
[0086] Targeted biological therapies hold tremendous potential for the treatment of cancers, yet their effective use has been limited by constraints on delivery and effective tumor targeting. Here we combine an immune effector cell population; cytokine induced killer (CIK) cells, with a complementary oncolytic viral therapy as an effective treatment of ovarian cancer. CIK cells, an ex vivo expanded population of cells derived from human peripheral blood, were used to deliver a replication competent vaccinia virus carrying genetic deletions that restrict its replication to malignant cells. Pre-infection of CIK cells with oncolytic vaccinia virus resulted in a prolonged eclipse period where the virus remained within the CIK cells until interaction with, and infiltration into, the tumor. In this combined therapeutic not only did the CIK cells retain their ability to traffic to ovarian tumors, but once at the tumor site the oncolytic virus was released deep in the tumor rather than mere...
example 2
[0102] The dual biotherapy as described above was effective in reducing tumor cell number for a variety of cervical cancer cells in vitro
[0103] A human cervical cancer cell line (SPEC) labeled with luciferase was tested in vivo. 5×106 tumor cells were implanted intraperitoneally into immunodeficient (SCID) mice and allowed to grow for 7 days. Mice were then treated with a single intraperitoneal injection of either PBS, 1×107 CIK cells or 1×107 CIK cells pre-infected with a TK and VGF double deleted vaccinia virus. CIK cells alone transiently delayed the growth of these tumors. Vaccinia virus alone reduced tumor burden, which was followed by relapse in 5 of 7 mice (i.e. 2 of the 7 mice displayed a complete response). The dual biotherapy reduced tumor burden, where 5 of the 7 mice displayed a complete recovery from the tumor.
example 3
[0104] A mouse non-small cell lung cancer cell line (CMT 64) was tested in immunocompetent C57B / 6 mice. Tumor cells were implanted subcutaneously, and allowed to grow until tumors reached 50-100 mm3. Animals were then treated with a single tail vein injection of either PBS, 1×107 PFU of TK deleted vaccinia virus or 1×107 CIK cells pre-infected with the same vaccinia virus. The dual biotherapy of the invention significantly increased the survival of these mice relative to virus alone (with median survival being 27 days in PBS treated mice; 38 days in virus treated mice and 59 days in dual biotherapy mice). 1 of 7 mice in dual biotherapy group displayed a complete recovery from the tumor, and none in the other treatment groups.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com