Liposome co-loaded with CD73 antibody and adriamycin, and preparation method and application of liposome
A doxorubicin and liposome technology, applied in the field of liposomes co-loaded with CD73 antibody and doxorubicin and their preparation, can solve problems such as drug resistance, reduce drug resistance and improve immune depletion state , the effect of promoting accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
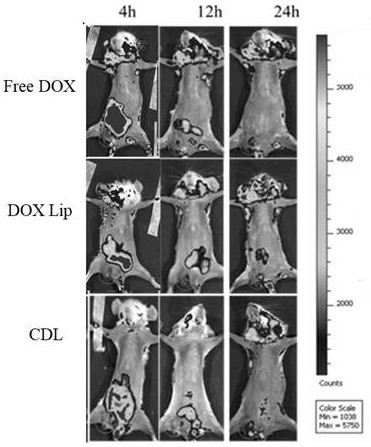
Image
Examples
preparation example 1
[0035] Preparation of immunochemotherapeutic liposomes (CDL) co-loaded with CD73 antibody and DOX
[0036] The CD73 antibody used in the examples of the present invention is a commercial product purchased from BioXcell Biotechnology Co., Ltd., USA.
[0037] 1. Preparation of DOX-loaded liposomes (DOX Lip)
[0038] First, dissolve the appropriate concentration of DOPC:DOPE:CHEMS= molar ratio 2:6:1 in 3mL of chloroform, rotary evaporate, the temperature of the water bath is 22°C, and the rotation speed is 80rpm / min, finally forming a transparent spiral After the lipid film, dry thoroughly to remove the solvent.
[0039] 2. Add an ammonium sulfate solution (250mmoL, pH=5.5) properly preheated to 40°C for ultrasonication in a water bath, and repeat extrusion several times with a micro liposome extruder. The obtained solution was put into a treated dialysis bag (3000 Da), stirred and dialyzed in PBS solution for 4 hours at a constant speed, and the dialysate was changed every 2 h...
Embodiment 1
[0042] Example 1 Research on CDL Release Behavior in Vitro
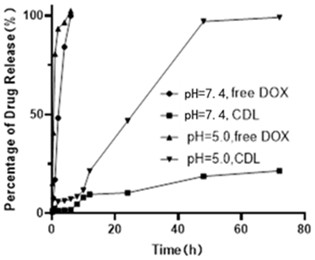
[0043] Experimental method: Put the same volume of free doxorubicin (free DOX) and CDL preparation (preparation example 1) in the dialysis bag, put all the samples in PBS and shake at constant temperature to simulate the in vivo environment, 37°C, 100 r / min, the sample solution was sampled at different time points (0.25, 0.5, 1, 2, 4, 6, 8, 10, 12, 24, 48, 72 h, 96 h), and the PBS medium with the same pH was added again. Pass the sample solution taken out through a 0.22 μm organic filter membrane, use HPLC to detect and record its peak area, and analyze the release behavior of the sample in vitro. The results of CDL release behavior in vitro are as follows: figure 1 shown.
[0044] Depend on figure 1 It can be seen that the release rate of doxorubicin in CDL is significantly reduced. At pH=7.4, the cumulative release rate of the preparation at 72h was 23.15±0.58%, showing a significant sustained release effect. ...
Embodiment 2
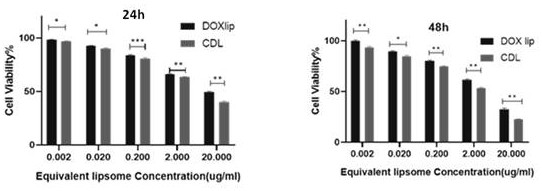
[0045] Example 2 The Killing Effect of CDL on Triple Negative Breast Cancer Cells
[0046] Cell killing experiment: triple-negative breast cancer cells (4T1 cells) were mixed with 4×10 4 cells / mL, 200 μL / well were inoculated in a 96-well plate, and cultured overnight to make them adhere to the wall. The experimental groups were grouped as follows: blank liposomes, doxorubicin liposomes (DOX Lip), liposomes (CDL) co-loaded with doxorubicin and CD73 antibody, and the control group was added with complete medium, which were converted into DOX concentrations ( 0.002, 0.02, 0.2, 2, 20μg / mL) were added to the corresponding 96-well plate; the blank liposome group was added to the corresponding well at the concentration of (0.02, 0.2, 2, 20, 200μg / mL), and 100μL was added to each well drug volume. Place it in the incubator for 24h / 48h after the operation is completed. Add the prepared CCK-8 solution (10 μL / well) to each sample well and place it in the incubator for 1 hour. The max...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com