Multifunctional fusion protein and application thereof
A fusion protein, multi-functional technology, applied in the field of fusion proteins, can solve the problems of low curative effect, achieve the effects of improving curative effect, improving inhibition and clearance, and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
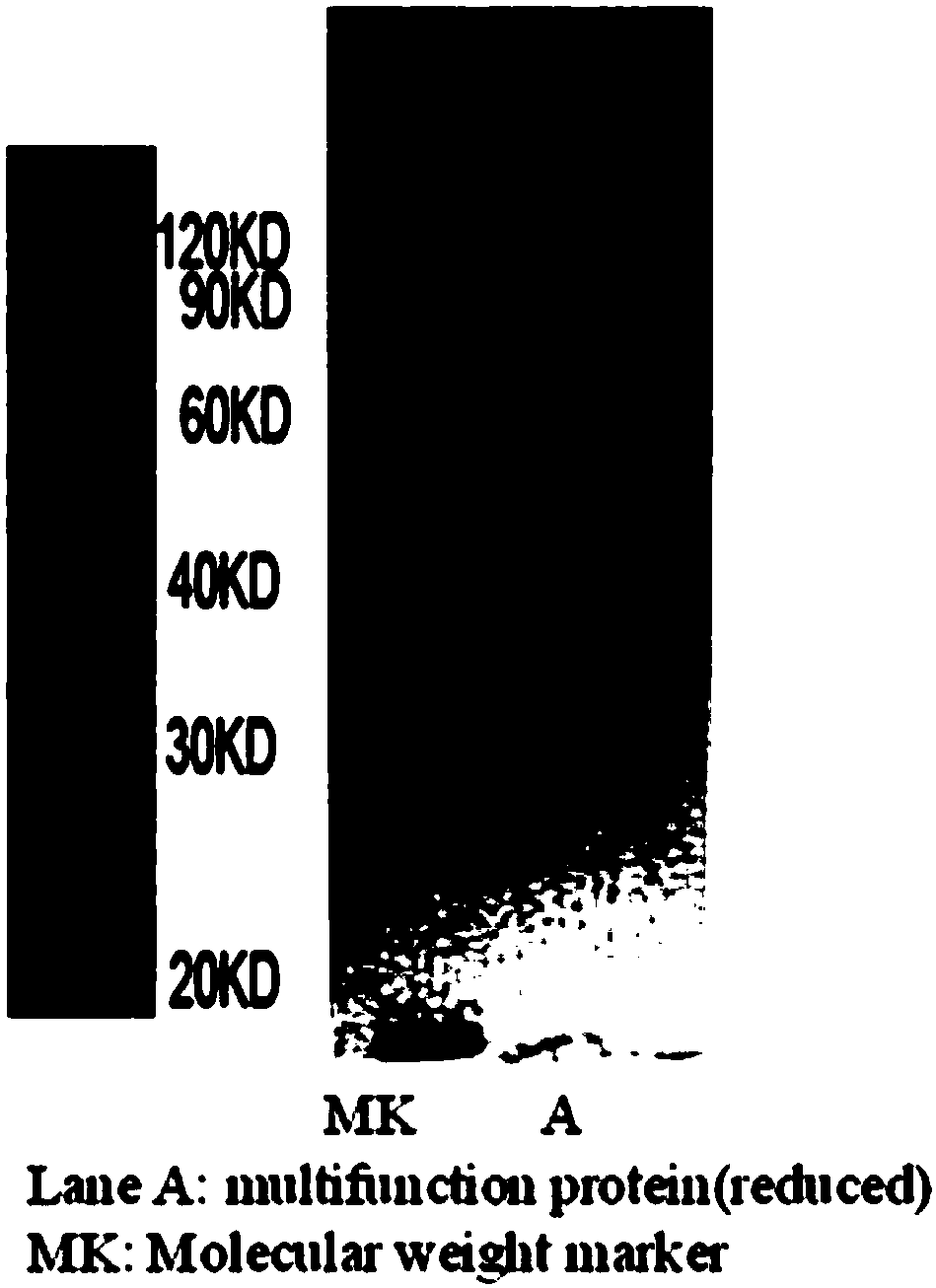
[0040] This example is the gene construction and production purification of the recombinant fusion protein.
[0041] According to the functional region bases of human SIRPα, IgG1Fc and PD-1, the multifunctional fusion protein (SEQ ID NO: 5) gene is formed by gene synthesis and non-functional amino acid flexible fragment bases, and then transferred into the eukaryotic expression vector pcDNA3 .1. Through gene digestion and further cloning, the multifunctional fusion protein gene is transferred into a eukaryotic animal expression vector. Finally, the vector of the fusion protein was transfected into Chinese hamster ovary cells (CHO). Transfected cells were placed at 37°C, 5% CO 2 Cultured in an incubator, the supernatant was taken after 72 hours, and further purified by ProteinA affinity chromatography, and the final purified protein was a multifunctional recombinant fusion protein. The molecular weight of the purified protein is confirmed by electrophoresis detection, which ...
Embodiment 2
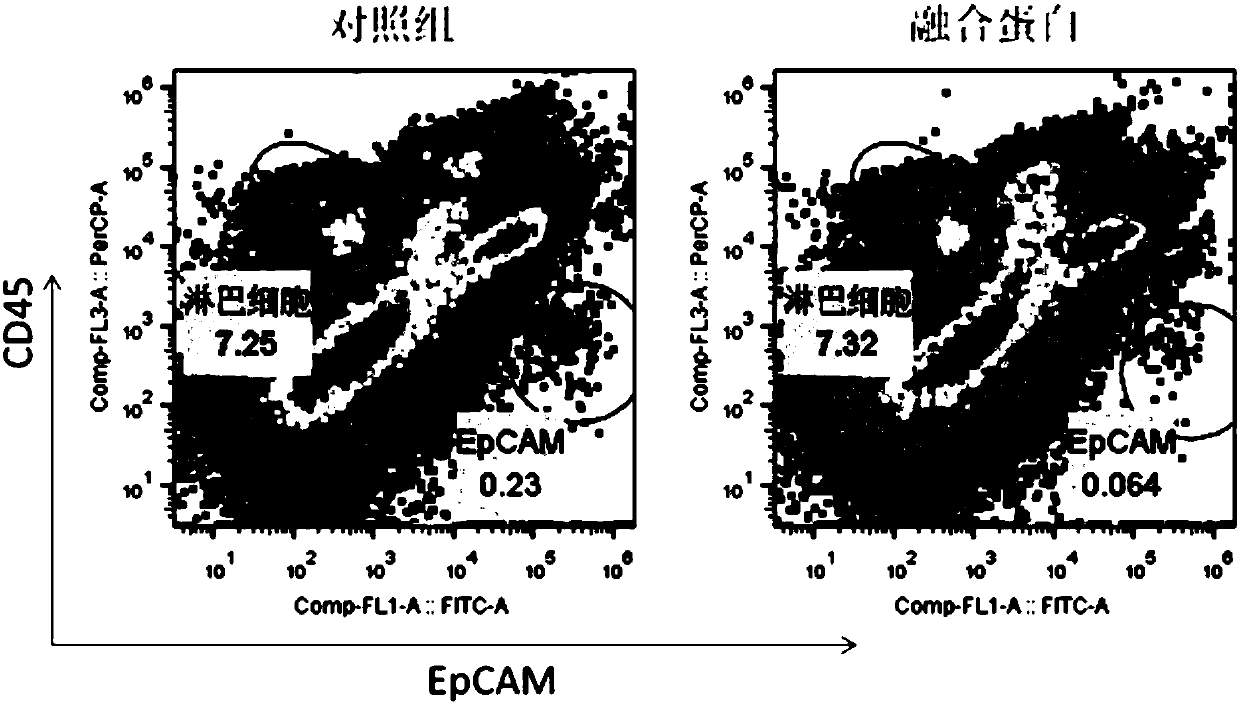
[0043] This example is the test of killing tumor cells in the ascites of patients with colorectal cancer by the multifunctional recombinant fusion protein.
[0044] Ascites of patients with colorectal cancer containing tumor cells and immune cells were collected and divided into two groups with 1ml per well and placed in a 24-well plate. The control group was added with PBS, and the experimental group was added with fusion protein to a final concentration of 1 μg / ml. After mixing, place at 37°C, 5% CO 2 Incubate for 48 hours in the incubator. After the cells were collected, they were washed once with PBS, and then stained with flow cytometry antibodies against immune cells (CD45) and tumor cells (EpCAM) for 20 minutes. After washing, flow cytometry was used for measurement and data analysis. figure 2 showed that compared with ascites of patients without fusion protein, fusion protein treatment can significantly reduce the proportion of tumor cells in ascites (0.23% vs 0.064%...
Embodiment 3
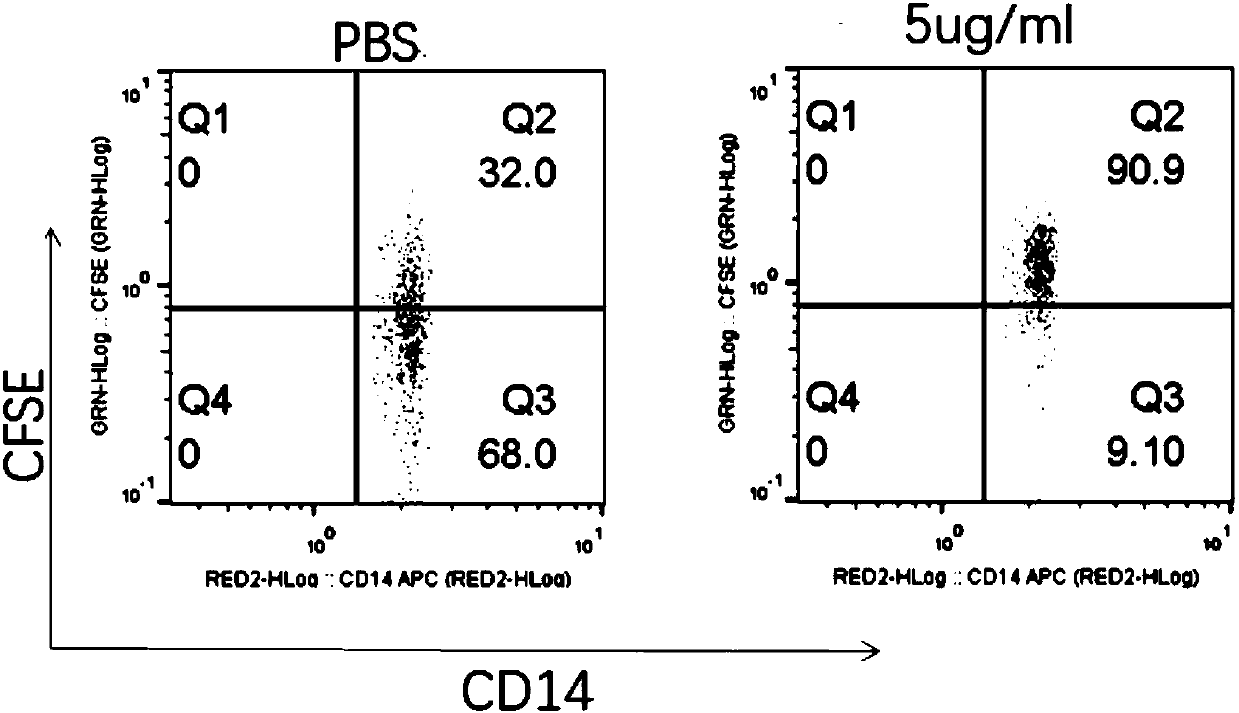
[0046] This example shows that the multifunctional recombinant fusion protein promotes the phagocytosis of tumor cells by macrophages in vitro.
[0047] 1x105 macrophages diluted in 100 μl DMEM medium were transferred to a 96-well plate, placed in a 37°C, 5% CO2 incubator for 2 hours, and then 2x105 tumor cells labeled with CFSE in 100 μl DMEM medium were added. Add fusion protein to a final concentration of 5 μg / ml as the fusion protein group, and wells without fusion protein as the control group. After continuing to culture for 2 hours, all the cells were collected, centrifuged, added anti-CD14 loss antibody, stained for 20 minutes, washed and analyzed by flow cytometry. image 3 The middle indicates that adding the fusion protein promotes the phagocytosis of H358 tumor cells. Compared with the control group, the proportion of CD14 cells containing CFSE tumors increased from 32% in the control group to 90.9% in the fusion protein group.
[0048] The above method was used to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com