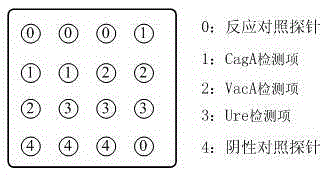
Protein chip for typing detection on helicobacter pylori infection
A Helicobacter pylori and protein chip technology, applied in measurement devices, analytical materials, biological tests, etc., can solve the problems of inability to judge type I and type II infections, limited positive rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Preparation of protein chip
[0019] Specifically include the following steps:
[0020] 1. Preparation of NC Membranes
[0021] (1) Soak the NC membrane with a pore size of 0.45 μm in distilled water first, take it out and dry it, soak it in 0.05mol / L carbonate buffer solution and then dry it in air.
[0022] (2) Cut the NC film into square pieces of 0.9 cm×0.9 cm with a semi-automatic film cutting machine, and set aside.
[0023] (3) Take the diluted 0.5mg / ml CagA antigen, 1.0mg / ml VacA antigen, and 1.0mg / ml Ure antigen and put them into the corresponding sample boxes in the spotting instrument respectively.
[0024] (4) Under the condition of temperature control (≤10°C), place the nitrocellulose membrane flatly in the spotting tank, and use the MicroGrid-2 spotting instrument to place the antigen solution, positioning solution, and quality control control solution in the form of a dot matrix. Form points on the nitrocellulose membrane, every 3 points as a...
Embodiment 2
[0034] Example 2 Preparation of a protein chip kit for typing and detecting Helicobacter pylori infection
[0035] The kit is composed of washing solution, chromogen and the protein chip of Example 1.
[0036] 1. Prepare washing solution
[0037] (1) Measure 500ml TBE buffer solution with a 1000ml graduated cylinder and pour it into a 2000ml beaker;
[0038] (2) Measure 100ml of saturated ammonium sulfate solution with a 100ml graduated cylinder, and pour it into a 2000ml beaker;
[0039] (3) Turn on the magnetic stirrer switch, adjust the rotating speed until the liquid can be fully stirred evenly;
[0040] (4) Measure 20ml Tween-20 with a 100ml measuring cylinder, pour it into a 2000ml beaker, and continue stirring;
[0041] (5) Measure 500ml deionized water with a 500ml graduated cylinder;
[0042] (6) Pour about 100ml into a measuring cylinder filled with Tween-20, shake the measuring cylinder to clean the residual Tween-20, and then pour into a 2000ml beaker;
[0043...
Embodiment 3
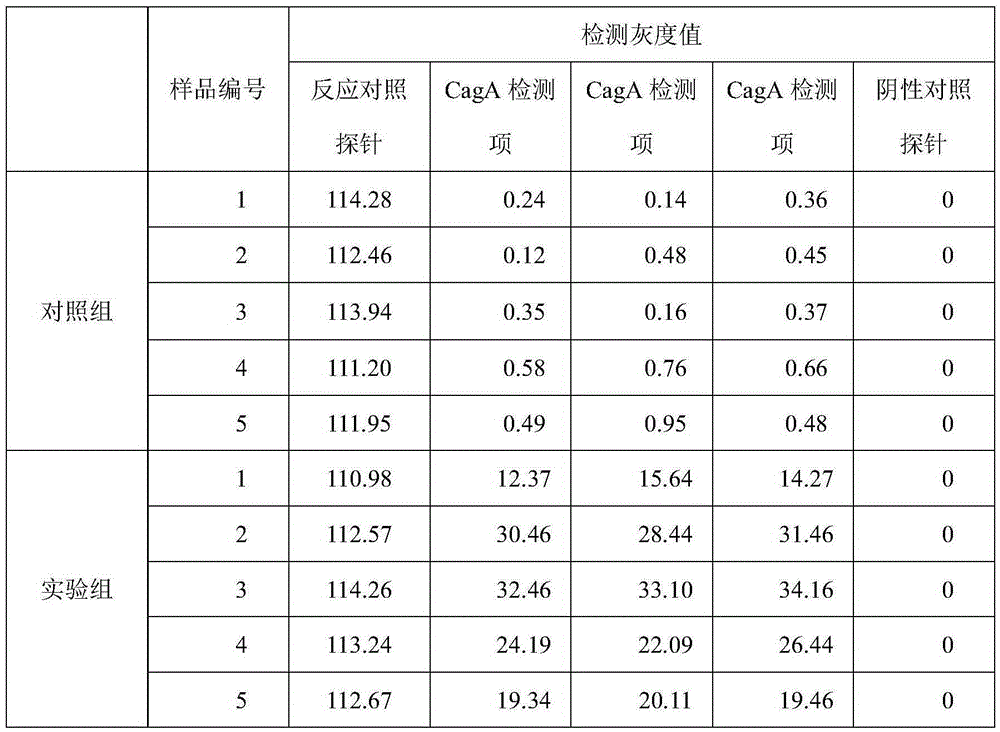
[0054] Example 3 Detection Verification
[0055] Five samples of serum from normal people and patients infected with Helicobacter pylori were collected and divided into control group and experimental group. Using the kit of Example 2, strictly follow the steps below to compare and verify the detection results of the protein chip of the present invention.
[0056] Detection steps:
[0057] 1) Moisturizing membrane: Take 160 μl (4 drops) of washing solution and add it to the membrane surface of the detection window of the chip to evenly wet the membrane surface;
[0058] 2) Adding samples: Take 200 μl of the processed serum to be tested and add it to the membrane surface of the detection window of the chip;
[0059] 3) Washing: After the sample is fully infiltrated, in order to ensure the washing effect, wash in two steps: take 120 μl (3 drops) of washing solution, add it to the membrane surface of the chip detection window, and after the washing solution penetrates, take anot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com