Schistosoma japonicum recombinant antigen, and preparation method and application thereof
A technology for recombinant antigens and schistosomiasis, applied in the field of bioengineering, can solve the problems of few reports and no research on protein characteristics, and achieve good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
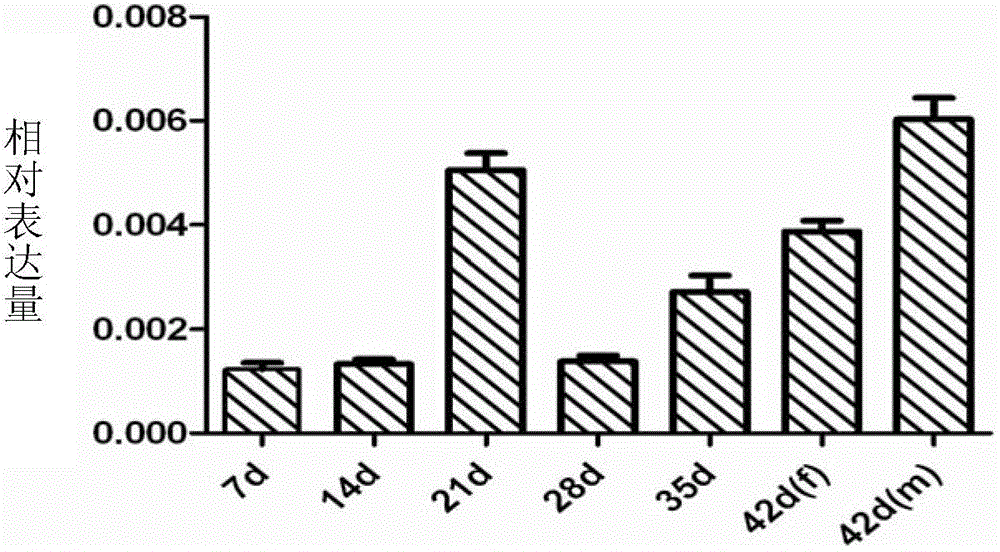
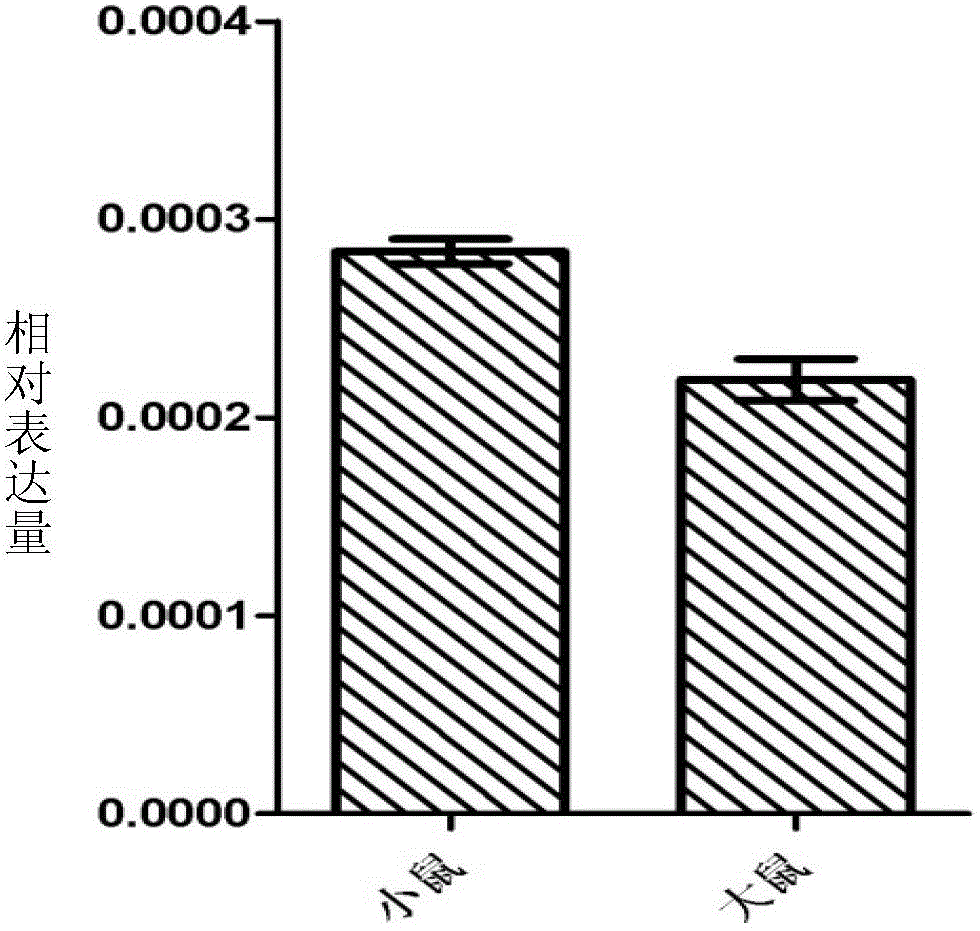
[0041] Example 1 Expression and Purification of Schistosoma japonicum Recombinant Antigen
[0042] 1. Method
[0043] 1.1 Construction of recombinant expression plasmids
[0044] According to the gene sequence of SiPGK with NCBI accession number FN322120.1, primers were designed, upstream primer: 5'-TAGGATCCATGGGTTTGTCGAAGCTCAG-3' (SEQ ID NO.3, containing BamH I restriction site); downstream primer: 5'-TCTCTCGAGTTAGTGAGCATCCGTAAG -3' (SEQ ID NO.4, containing Xho I restriction site). Using the cDNA of Schistosoma japonicum 42-day-old body as a template, PCR amplifies its cDNA fragment containing ORF, and the reaction composition is as follows:
[0045]
[0046]
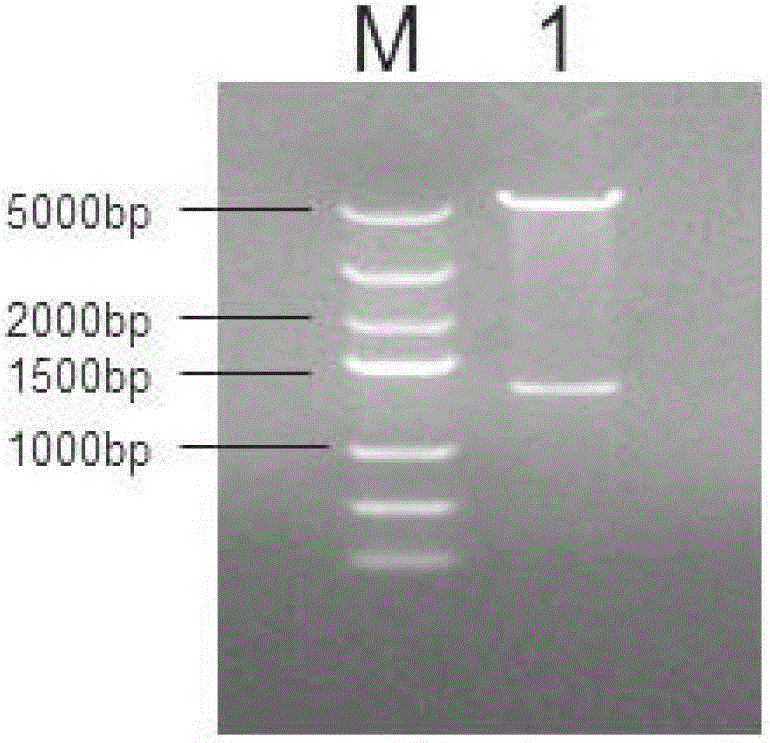
[0047] PCR reaction conditions: 94°C for 5min, denaturation at 94°C for 30sec, annealing at 58°C for 90sec, extension at 72°C for 90sec, a total of 30 cycles; finally 72°C for 10min. After the PCR product was purified, it was connected to the pMD19-T vector, transformed into DH5α cells, and a single clone was ...
Embodiment 2
[0065] Example 2 Detection of Enzyme Activity of Schistosoma japonicum Recombinant Antigen rSjPGK
[0066] The reaction system for rSjPGK to catalyze 1,3-DPG to generate 3-PGA is: 0.1M triethanolamine (pH7.6), 5mM MgSO 4, 0.5mM EDTA, 1.0mM ATP, 0.2mM NADH, 6mM 3-PGA, 4U / mL GAPDH. Adjust the pH value of the assay buffer to 7.6, 8.0, 8.2, 8.4, 8.6, 8.8, 9.0 (0.05M Tris-cl buffer), 9.5, 10.0, 10.5 (0.05M glycine-sodium hydroxide buffer). Then add other reagents in the reaction system, incubate at 25°C for 5 minutes, add the recombinant protein rSjPGK, measure the absorbance change at 340nm for 1 minute in each system, and repeat the experiment three times. Take the highest activity as 100%, calculate the ratio of the activity of other pH values relative to the highest activity, take pH as the abscissa, and relative activity as the ordinate to make a reaction curve.
[0067] Adjust the temperature of the reaction system to eight different gradients of 10°C, 15°C, 20°C, 30°C, 3...
Embodiment 3
[0071] Example 3 Antigenic Detection of Schistosoma japonicum Recombinant Antigen rSjPGK
[0072] 1. Western Blotting analysis of recombinant protein antigenicity
[0073] Perform SDS-PAGE electrophoresis on the recombinant protein rSjPGK and Schistosoma japonicum 14d larval protein after Ni column purification, then transfer to NC membrane at 4°C with 130mA for 1h, and use anti-recombinant protein antiserum as the primary antibody Antigenicity analysis of recombinant proteins.
[0074] 2. Western Blot analysis of recombinant protein antigenicity results
[0075] The results of Western Blot analysis showed that the recombinant protein rSjPGK immune serum could recognize the recombinant protein and the natural worm protein in the 14d worm protein (as a natural protein of PGK, it is a positive control), indicating that the recombinant protein rSjPGK has good immunogenicity ( Figure 9 ). exist Figure 9 Middle, A: M: Marker; 1: recombinant protein rSjPGK as antigen; B: M: M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com