Fusion protein and application thereof in preparation of novel coronavirus subunit vaccine
A fusion protein and virus technology, applied in the field of vaccines, can solve the problems of weak immunogenicity and enhanced antibody-mediated diseases, and achieve the effect of prolonging the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
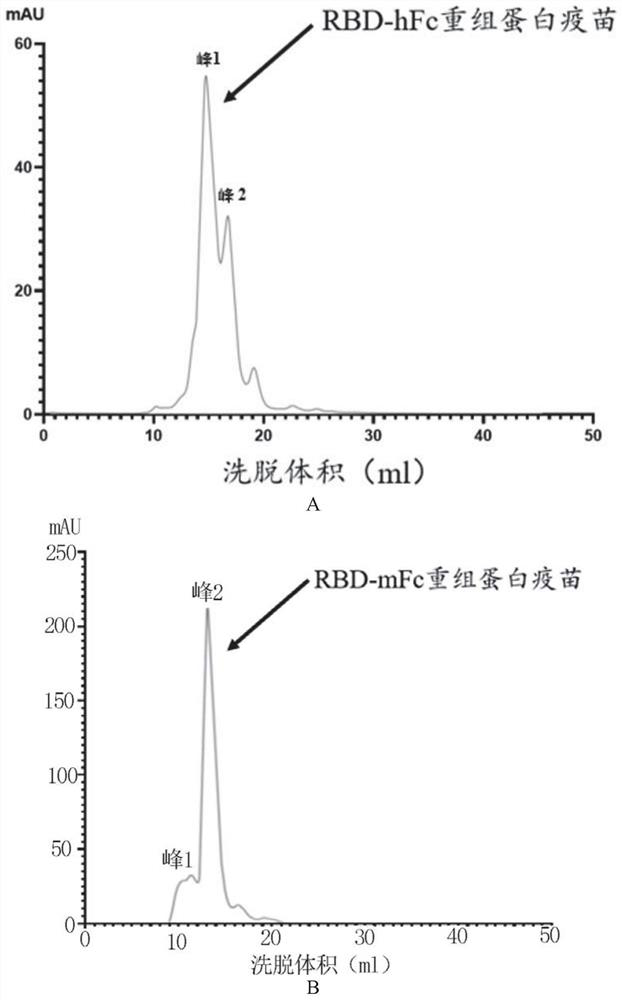
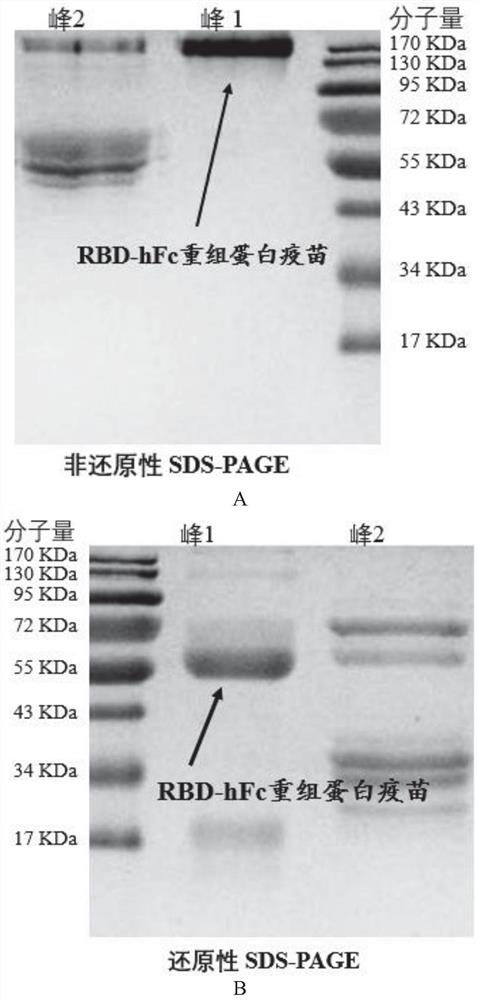
[0026]Example 1: Preparation of RBD recombinant protein
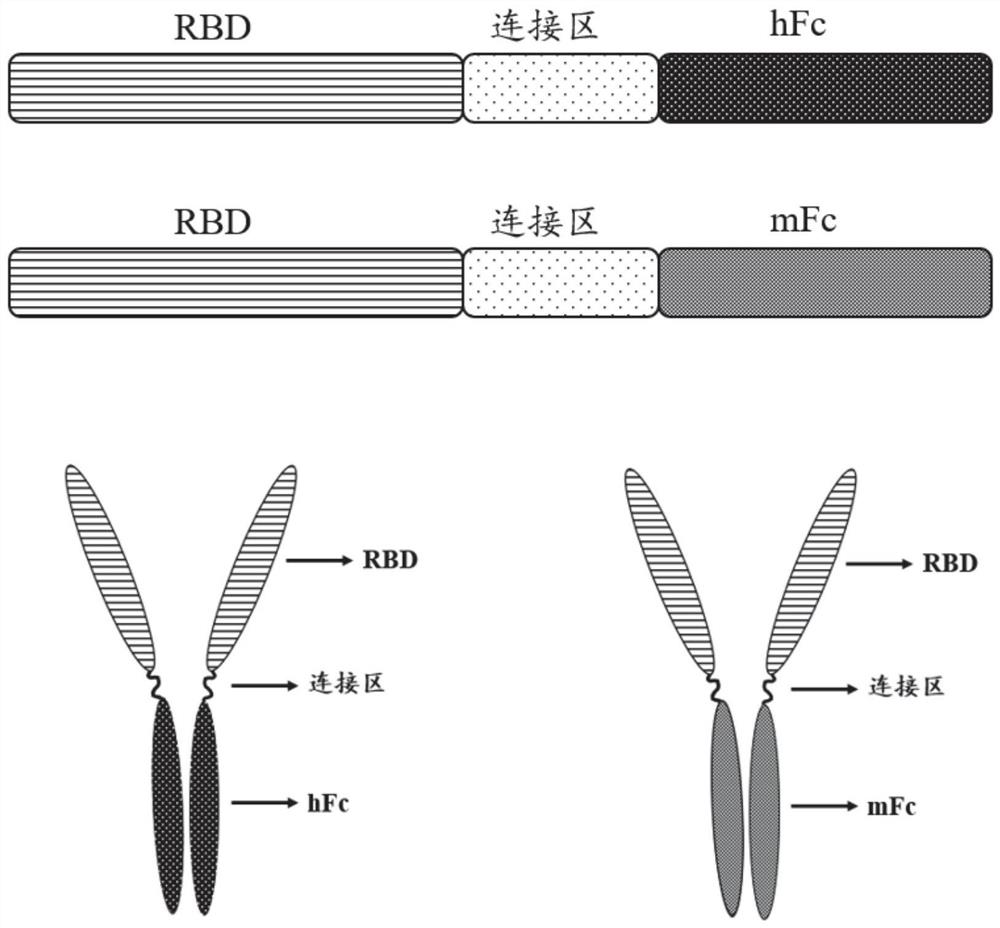
[0027](1) The RBD sequence is derived from the SARS-COV-2 penetration protein 330-583 amino acid range. In the present invention, the source Fc from the mouse source IgG1, the human source Fc is the transformation on the basis of human IgG1. The specific transformation site of human source FC is in ASN297ala and Lys322ala, and the ADCC and CDC effects mediated by FC are redundant. In order to increase the safety of the vaccine, the two sites are mutated.
[0028]Experimental Design Route: First PCD (Gene Sequence SEQ ID No.4, Amino Acid Sequence SEQ ID NOT), and Human IgG1 modified Fc fragment (hereinafter simply referred to as HFC, gene sequence SEQ ID NO) 5, amino acid sequence SEQ ID No. 2), the Fc fragment (hereinafter simply referred to as MFC, gene sequence SEQ ID NO. 6, amino acid sequence SEQ ID NO.). By overlapping the PCR, RBD and HFC and MFC are spliced through a flexible connection zone (Gly-Gly-Gly-ser, gene sequence...
Embodiment 2
[0031]Example 2: Flow affinity determination
[0032](1) Collection 1 × 106VERO E6 cells were respectively composed of RBD-HFC recombinant protein vaccine (10 μg / ml), RBD-MFC recombinant protein vaccine (10 μg / ml), and two groups of negative controls (only PBS only). It was incubated at 4 ° C for 30 min.
[0033](2) After washing of PBS was 3 times, the second anti-rats of Fitc labeled goats were added to the Test tube of RBD-MFC; and the addition of FITC labeled goats were added to the test tube of RBD-MFC. Second Anti-(1: 1000) 200 μL; the negative control group, added to the Fitc labeled goat anti-human secondary antibody (1: 1000) 200 μL, FITC-labeled goat anti-mouse two-resistance (1: 1000) 200 μL. After incubation of 4 ° C for 30 min, PBS was washed 3 times, and the flow cytometer was detected.
[0034](3) Flow cytometry detection of affinity of RBD recombinant protein and Vero E6 cells (ACE2 positive) is mainly expressed by the average fluorescence intensity of FITC after binding....
Embodiment 3
[0036]Example 3: Recombinant protein immunization mice
[0037]Choosing healthy BALB / C female mice, 8 weeks (19-21g), mice were randomly divided into 7 groups, and 4 of each group, F, G, H, I, J, K, L total 7 groups, Specifically, the F group is inoculated by Al (OH)3Adjurant group; Group g is a high dose (8 μg) RBD-HFC (RBD-HFC)HIGH ), H group is a low dose (2 μg) RBD-HFC (RBD-HFC)Low); Group K is inoculated with high dose (8 μg) RBD-MFC (RBD-MFC)HIGH ), Group L is a low dose (2 μg) RBD-MFC (RBD-MFC)Low). Further, in order to compare the immunogenic differences in the recombinant protein vaccine in the two Fc fusion forms of RBD and S1, S1 recombinant protein vaccine fusion human source Fc is provided as a control, S1-HFC (purchased from Beijing Yiqiao Shenzhou Company, Item No. 40591-V02H) In the S1 domain selection S protein 16-685AA, where the Fc is a human source IgG1 (ASN297, and the Lys322 are not mutated).
[0038]Specific as follows: I group is inoculated with high dose (8 μg) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com