Anti-novel coronavirus antibody and preparation method and application thereof
A coronavirus, a new type of technology, applied in the field of anti-new coronavirus antibodies and its preparation, can solve the problems of limited auxiliary role in the prevention and control of group viruses, inability to achieve specific killing of pathogens, and high degree of dependence on the external environment. It is not easy to achieve Allergic reactions, wide range of protection, and the effect of less dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
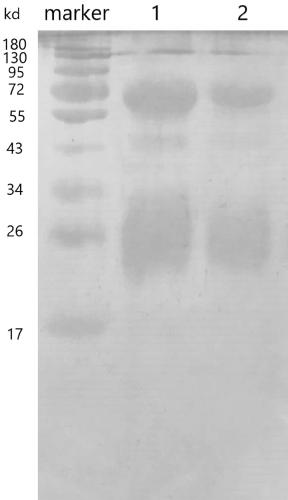
Image
Examples
Embodiment 1
[0028] The present invention specifically provides an anti-new coronavirus antibody, which is characterized in that the antibody is a high-titer specific antibody extracted from chicken egg yolk, and the chicken egg yolk is mainly obtained from eggs produced by immunized hens. Obtained, the immunized hen is a healthy hen injected with a new coronavirus vaccine.
[0029] Wherein, the novel coronavirus vaccine is mainly prepared from recombinantly expressed novel coronavirus (SARS-CoV-2) S antigen or virus-inactivated novel coronavirus (SARS-CoV-2) antigen.
[0030] Its specific preparation method comprises the following steps:
[0031] 1. Antibody preparation:
[0032] S1. Vaccine preparation:
[0033]Take the novel coronavirus (SARS-CoV-2) S antigen expressed recombinantly or the virus-inactivated novel coronavirus (SARS-CoV-2) antigen, and add the antigen into Freund's complete adjuvant in a ratio of 1:1 (initial immunization) or Freund's incomplete adjuvant (boosting immu...
Embodiment 2
[0056] This embodiment compares the yolk antibody production situation of two groups of different antigen immunization doses, and the preferred antigen immunization dose is specifically shown in the following table:
[0057] Table 1 The rise and fall of egg yolk antibody titers with different antigen immunization doses
[0058] immune dose 4 weeks after priming 5 weeks after priming 6 weeks after priming 8 weeks after priming 10 weeks after priming 5μg / feather 1:16000 1:80000 1:80000 1:80000 1:64000 10μg / feather NT 1:40000 1:40000 1:80000 1:32000
[0059] (NT: As the hens did not lay eggs after immunization, no detection was performed.)
[0060] It can be seen from the above table that the 5 μg / feather antigen dose group is preferred, and the yolk antibody titers produced in this dose group after 6, 8, and 10 weeks of primary vaccination are not lower than the 10 μg / feather antigen dose group.
Embodiment 3
[0062] In this embodiment, in order to verify the anti-new coronavirus yolk antibody neutralizing virus performance, the following tests were carried out:
[0063] take 10 5 ~10 7 TCID 50 The new coronavirus liquid per ml is mixed with the new coronavirus egg yolk antibody samples with a concentration of 1mg / ml, 2mg / ml, and 3mg / ml according to the volume ratio of 1:1, and then neutralized at 25-37°C for 1-2 hours. Add it to a 96-well plate containing cells, incubate in a carbon dioxide incubator at 33-35°C, and observe cell changes daily under a microscope.
[0064] Under the condition of 25-37°C, the new coronavirus yolk antibody with a concentration of 2mg / ml and 3mg / ml was mixed with the new coronavirus liquid of 105-107 TCID50 / ml, and the cells had no pathological changes. As a positive control, the novel coronavirus liquid can cause pathological changes in cells.
[0065] It can be seen from the test results that the anti-new coronavirus IgY prepared in the present in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com