Novel coronavirus vaccine and preparation method and application thereof
A coronavirus and vaccine technology, applied in the field of biomedicine, can solve the problem of no treatment for pneumonia, and achieve the effect of enhancing immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089]Example 1 Design and synthesis of coding genes of fusion proteins
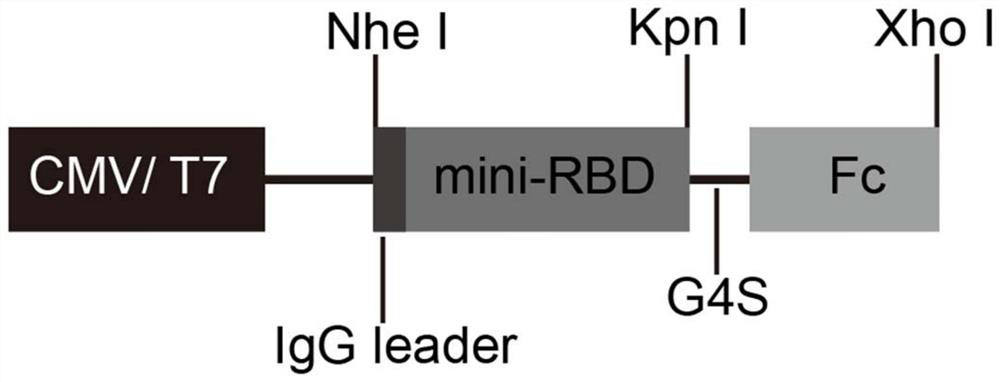
[0090]Section 1168 to 1569 Base base from the CDS coding sequence from Spike Protein is selected as a gene sequence (SEQ ID NO: 7) that contains a clip of a key binding site that binds to the ACE2 in the RBD, and is modified: add it to the 5 'end. Kozak sequences to increase the expression of proteins, while adding IgG secreted signal peptide sequences to enhance proteins, adding human IgG FC domain sequences (Fc (HOMO) to enhance protein immunity at 3 'ends The original, G4S is provided between the s protein receptor binding domain sequence and the human IgG Fc domain sequence, and the encoding gene of the antigen shown in SEQ ID NO: 9 is configured;
[0091]Or optimized objects in SEQ ID NO: 7, in accordance with codon preferences, select the highest frequency codon using the highest frequency, using Kesray OptimumGene codon optimization and genetic design technology (https: / / www.genscript.com.cn / / CODON_OPT_PR.HT...
Embodiment 2
[0093]Example 2 Construction of recombinant carrier
[0094]Add a NHE I enzyme dug point at the 5 'end of the encoding gene shown in SEQ ID NO: 9 or SEQ ID NO: 10, and the 3' end is added to add XHO I enzyme, using restriction endonase NHE I and XHO I Digestive Coding Gene, with Omega EZNAGelextraction Kit purification recycling;
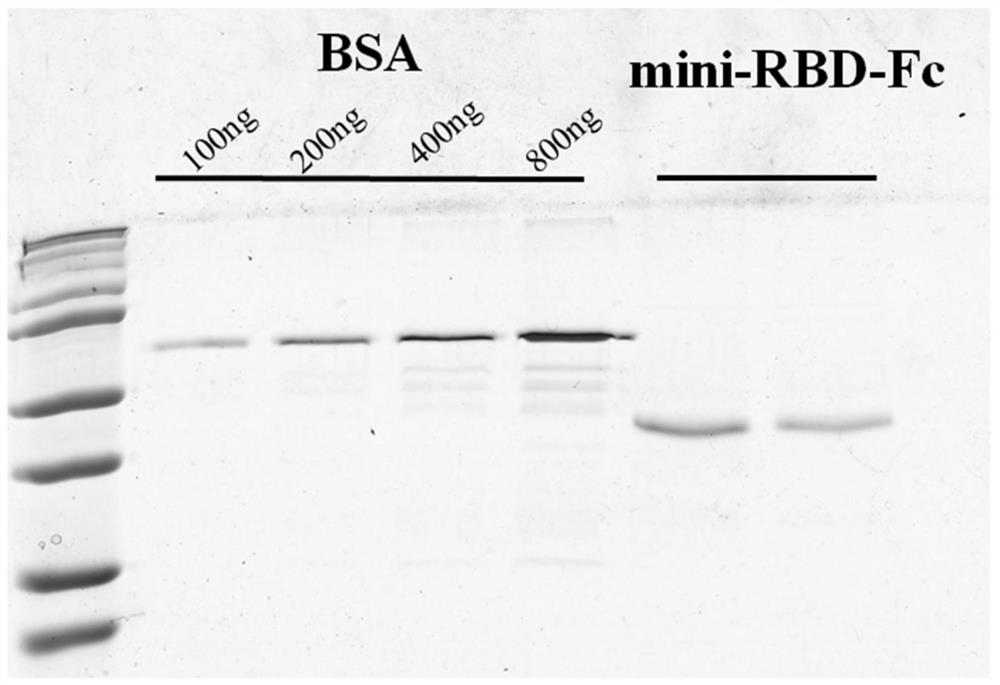
[0095]Also use restriction endonuclease NHE I and XHO I digestion plasmid pcDNA3.1 (+), agarose gel electrophoresis, with omega e.z.n.aGel Extraction Kit recycled linear carrier, as a skeleton; use T4 DNA lid enzyme will be recycled, a linear carrier and encoding gene are connected to obtain recombinant carrier PCDNA3.1-mini-RBD-Fc, and a brief showing of recombinant carrierfigure 1 Indicated.
Embodiment 3
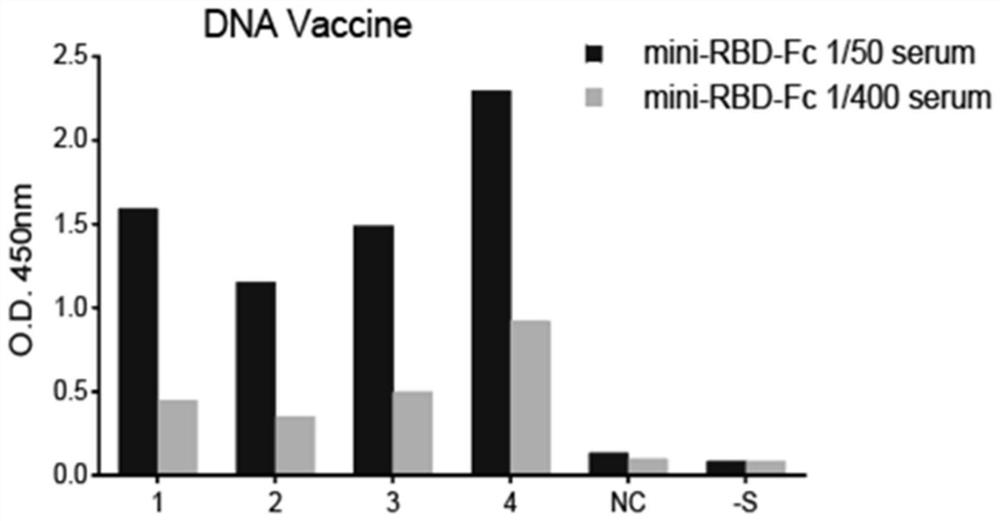
[0096]Example 3 Preparation and animal immunity of DNA vaccine
[0097]100 μg of recombinant carrier PCDNA3.1-mini-RBD-Fc was diluted with physiological saline to a final concentration of 1 μg / μL, and an immunization experiment was carried out as a DNA vaccine injecting mice:
[0098]After weighing the surrounding aged BALB / C mice, it was randomly divided into 2 groups (a set of experimental groups for injection plasmids, a group of control groups for injection of physiological saline), 4 per group, using abdominal injection to small Rats were anesthetized and the injected dose was 1% pentobarbital sodium 50 μl / 10g mice. After a anesthesia of 5-10 min, 100 μL of the back leg of the hind of each mouse was injected with 100 μL of recombinant carrier PCDNA3.1-mini- The RBD-Fc solution was immunized, and the first injection was injected again in the same conditions in the first injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com