A new pectinase gene and its protein expression, carrier and application
A pectinase and gene technology, applied in application, genetic engineering, plant genetic improvement and other directions, can solve the problems of low expression and few reports, and achieve the effect of strong biological activity, cost reduction, and mass production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
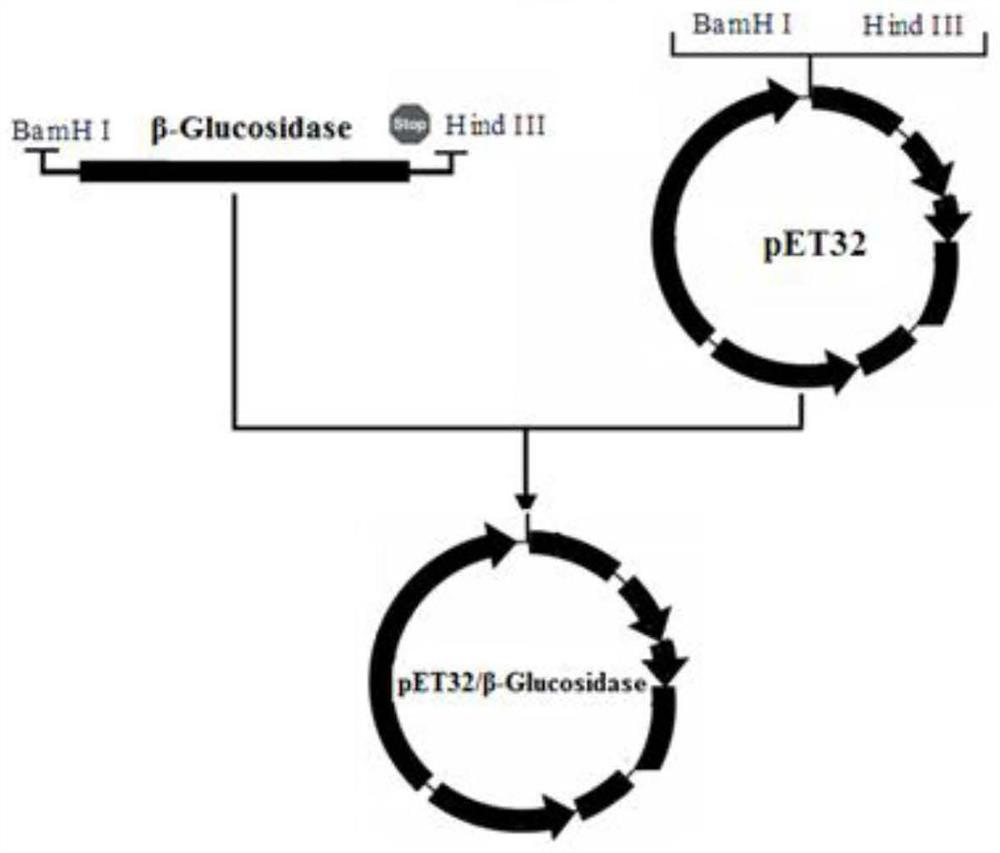
[0040] This embodiment provides an optimized artificially synthesized new pectinase gene, the specific sequence is shown in SEQ ID NO: 1 in the sequence listing, and the protein sequence corresponding to the gene is shown in SEQ ID NO: 2 in the sequence listing shown. The sequence provided by the present invention has no sequence with a similarity of 60% in the NCBI database. It is based on the characteristics of Escherichia coli expression such as codon bias, preventing complex DNA structures from affecting transcription efficiency, ensuring reasonable GC content, Select a DNA sequence among many sequences that are artificially optimized and synthesized with the characteristics of suitable restriction site, ideal expression tag and termination signal. The sequence described in the present invention and the highly homologous DNA sequence have higher expression of soluble target protein in Escherichia coli than other sequences.
[0041] The formation of Poria cocos is that the...
Embodiment 3
[0054] The present embodiment provides a kind of method for preparing pectinase protein, specifically comprises the following steps:
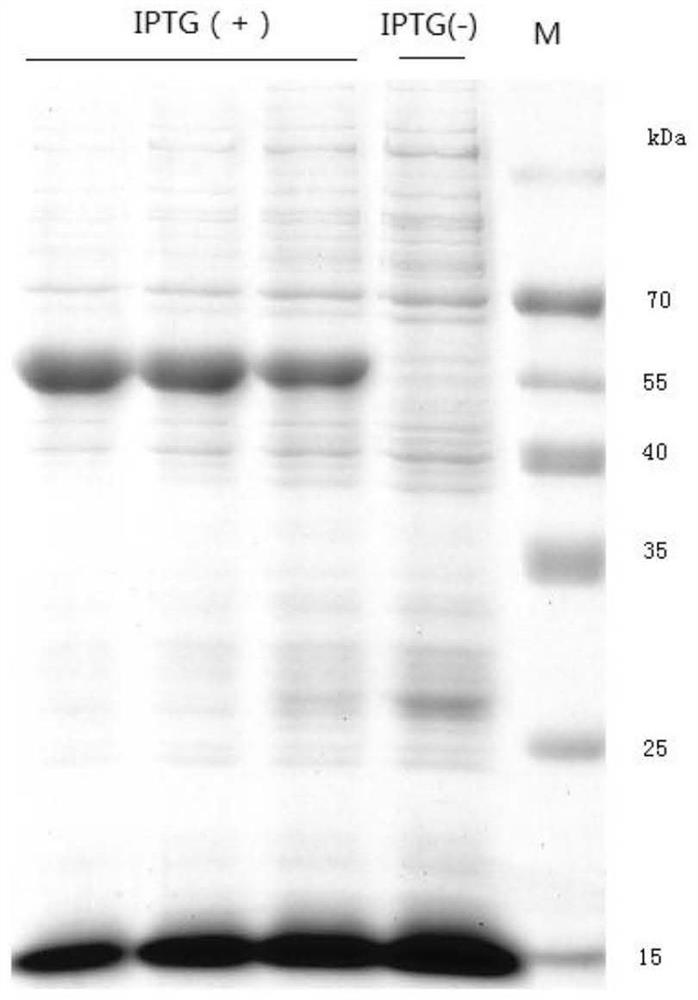
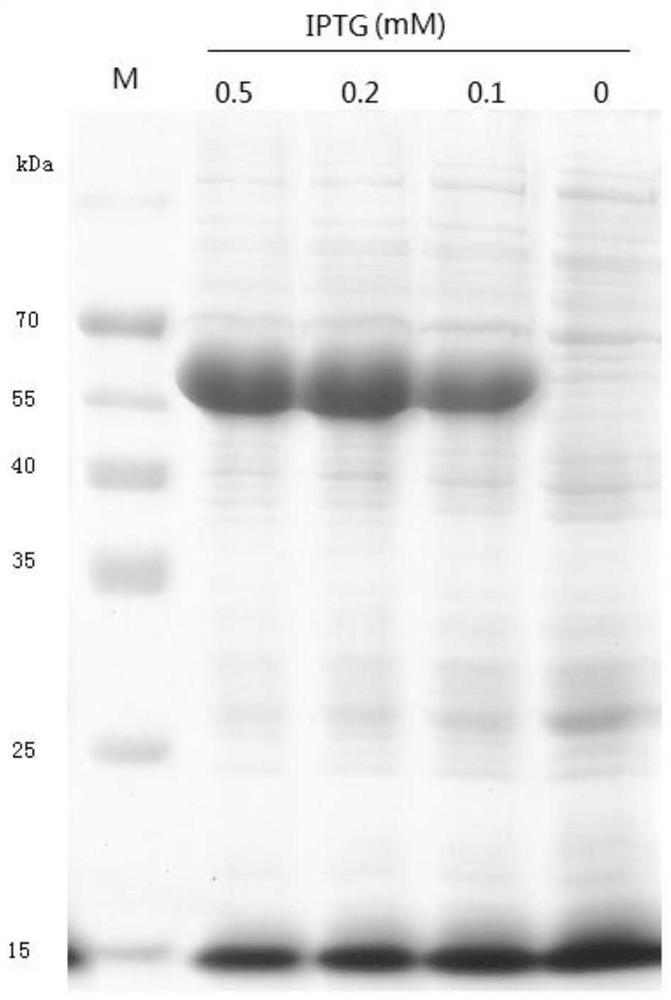
[0055] S1: Expression and extraction of soluble pectinase protein: the Escherichia coli recombinant transformant containing the pET32 / pectinase vector containing the sequence SEQ ID NO: 1 gene was cultivated in liquid LB medium at 37°C until the OD600 was 0.6, and then Add IPTG with a concentration of 0, 0.1, and 0.5 mM respectively, and induce at 18°C for 10 hours. After the induction, the collected bacteria are ultrasonically crushed, the crushing power is 300W, the crushing is 2s, the gap is 8s, and after 90 cycles, the supernatant is obtained by centrifugation. Obtain the soluble fusion protein pectinase of reorganization, SDS-PAGE result is as follows image 3 As shown, there was no significant difference in the expression of the target protein when induced by IPTG with a final concentration of 0, 0.1, and 0.5 mM. In order to save costs,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com