Group A, group B and group C meningococcus combined vaccine and preparation method thereof
A meningococcal and combined vaccine technology, applied in bacteria, antibacterial drugs, pharmaceutical formulations, etc., can solve problems such as hidden safety hazards of vaccines, reversal of toxicity of protein carriers, etc., and achieves broad targeting, good specificity, and good specificity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1AB
[0033] Embodiment 1ABC group meningococcal combined vaccine and its preparation process
[0034] This example is composed of purified component antigens from four strains of meningococcus, and the specific serogroups and antigenic components are as follows:
[0035] Group A meningococcal strains were obtained from China Medical Bacteria Collection Center (CMCC), strain number CMCC29201, and the component antigen was capsular polysaccharide (CPS).
[0036] Group C meningococcal strains were obtained from China Medical Bacteria Collection Center (CMCC), strain number CMCC29205, and the component antigen was capsular polysaccharide (CPS).
[0037] Group B meningococcal strains are from China Medical Bacteria Collection Center (CMCC), strain number CMCC29356, and the component antigen is outer membrane protein vesicle (OMV).
[0038] The group B meningococcal strain is derived from the isolated strain xrsw341215 from the blood samples of domestic group B meningococcal meningitis ...
Embodiment 2
[0054] Example 2 Comparison of Immunogenicity of Group AC Meningococcal Conjugate Vaccines from Different Manufacturers
[0055] Experimental program:
[0056] 1. Vaccines from four sources are domestic 1AC vaccine, domestic 2AC vaccine, Xiangrui biological AC vaccine and the ABC group meningococcal combined vaccine prepared in Example 1.
[0057] 2. Experimental animals: BALB / C mice, 12-14 grams / body weight.
[0058] 3. Grouping: 10 mice in each group, half female and half male, and 5 mice of the same sex were raised in each tank.
[0059] 4. Immunization method: subcutaneous injection; immunization dose: 2.5 μg polysaccharide / groups A and C.
[0060] 5. Immunization procedure: three injections in the whole process, that is, one injection each on day 0, 14, and 21.
[0061] 6. Serum separation and detection: Blood was collected two weeks after each immunization, serum was separated, antibody titer of a single mouse was determined by ELISA, geometric mean titer (GMT) of eac...
Embodiment 3
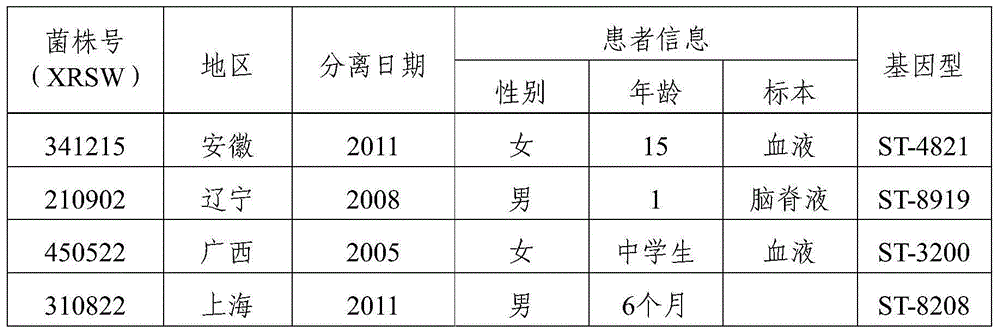
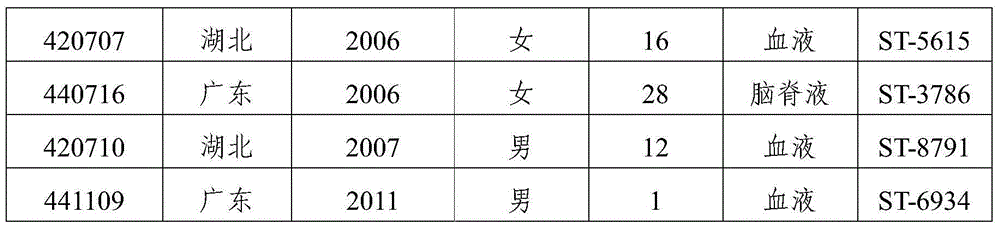
[0067] Example 3 Determination of the cross-antibody between the immune serum of xrsw341215 isolated from domestic group B meningococcal meningitis patients and dominant pathogenic group B meningococcal meningitis strains from different sources at home and abroad
[0068] 1. Domestic strains: Screened from 167 domestically collected ECM patients and carriers of group B, and identified 8 dominant strains in total (Table 2).
[0069] Table 2 Domestic isolates of group B patients
[0070]
[0071]
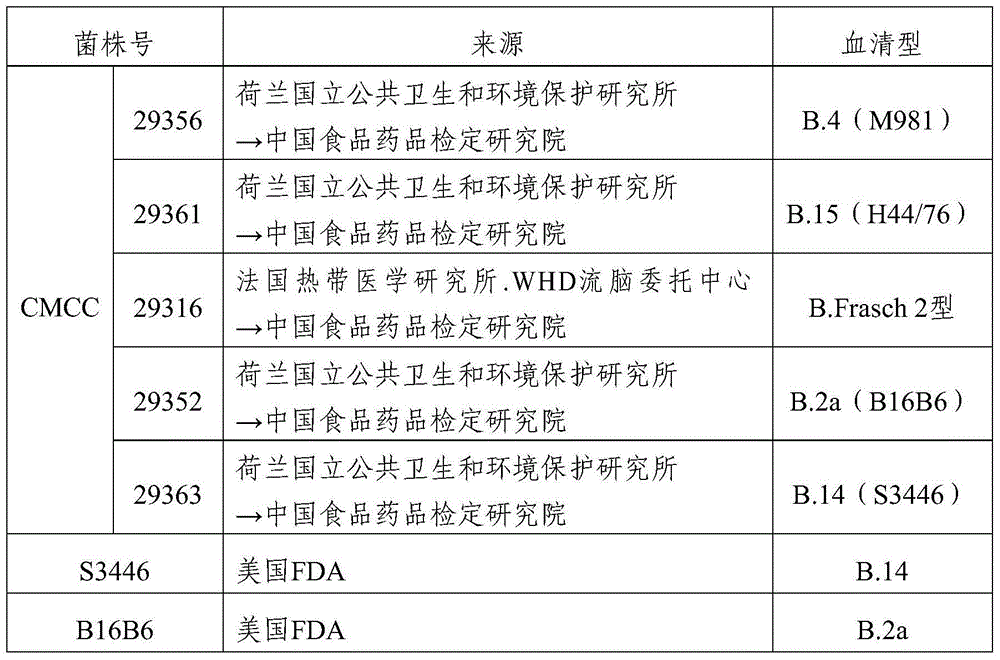
[0072] 2. Foreign strains: respectively from the US FDA, the Netherlands National Institute of Public Health and Environmental Protection, and China National Institute for Food and Drug Control. (table 3)
[0073] Table 3 Strains collected abroad
[0074]
[0075] 3. Preparation of immune serum of xrsw341215 strain
[0076] Animals: SPF grade N1H mice, 10 / group, 12-14 grams.
[0077] Preparation of immune stock solution: Kaikai working seed batch 1 strain, inoculated in s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com