Glycoside hydrolase family 7 protein gene and its encoded protein and application
A glycoside hydrolase, family technology, applied in the fields of application, sugar production, genetic engineering, etc., can solve few problems such as endocellulase and achieve good enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]This example provides an optimized artificially synthesized glycoside hydrolase family 7 protein gene (GH7), the specific sequence is shown in SEQ ID NO.1 in the sequence listing, and the protein amino acid sequence corresponding to the gene is shown in the sequence listing Shown in SEQID NO.2. The synthesized sequence has no sequence with a similarity of 70% in the NCBI database. It is optimized and synthesized according to the characteristics of E. coli expression.
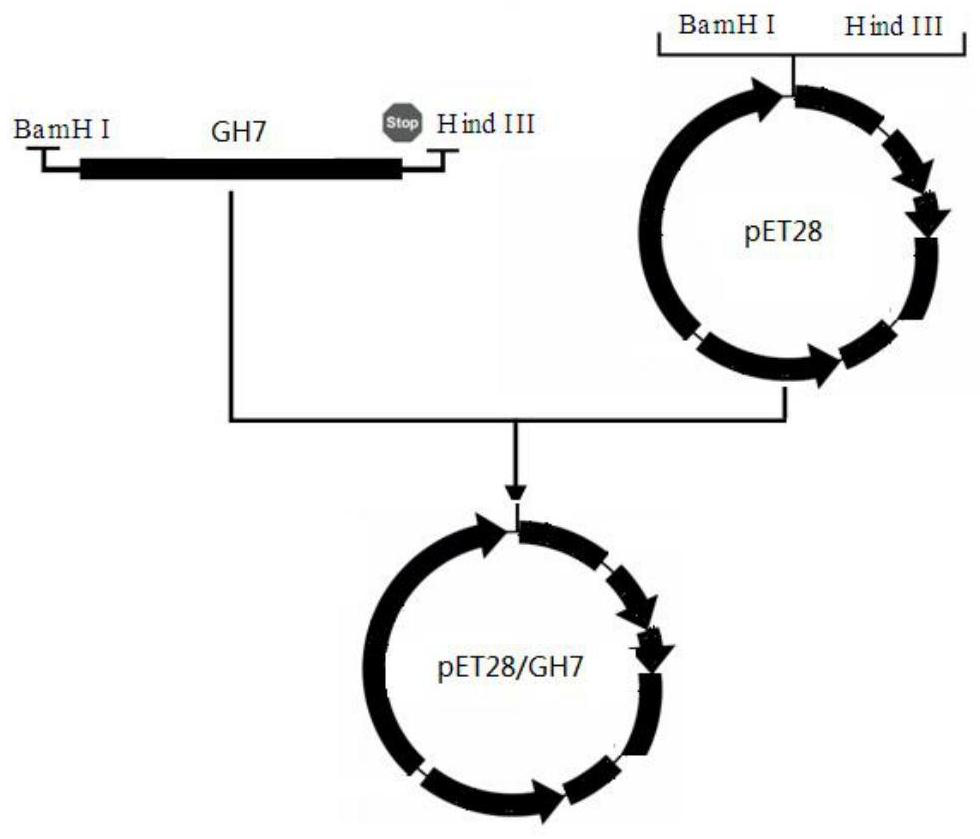
[0044] Connect the above-mentioned optimized natural gene into the E. coli expression vector pET28 to obtain the recombinant vector. The above-mentioned recombinant vector verified by sequencing is transformed into the competent cells of the E. coli expression strain by heat shock, and the corresponding resistant LB plate is coated, 37 Cultivate in a constant temperature incubator for 12 hours, and screen transformants, wherein the pET28 / GH7 vector is constructed as follows: figure 1 as shown, figure 1 I...
Embodiment 2
[0055] This embodiment provides a method for glycoside hydrolase family 7 protein, which specifically includes the following steps:
[0056] S1: Transform the recombinant vector pET28 / GH7 into Escherichia coli TOP10 strain, then extract the recombinant vector pET28 / GH7 from TOP10; transfer the recombinant vector pET28 / GH7 into the host cell E. The transformants of E. coli expressing strains containing the recombinant vector pET28 / GH7 were screened on the Kan-resistant LB plate, and the recombinant transformants were verified by PCR.
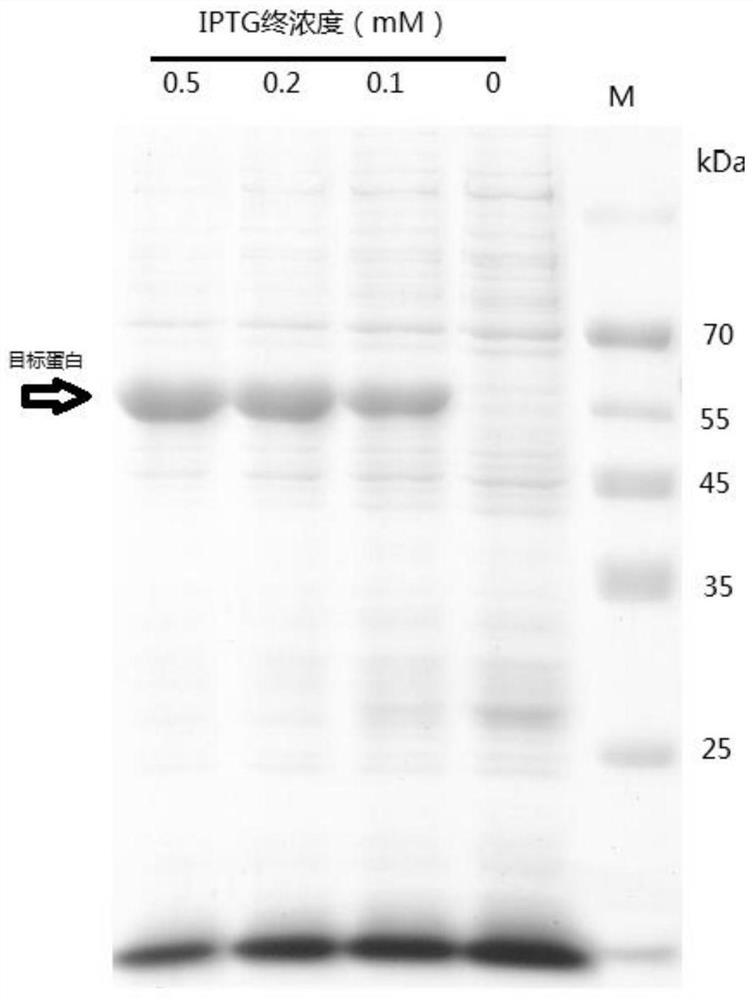
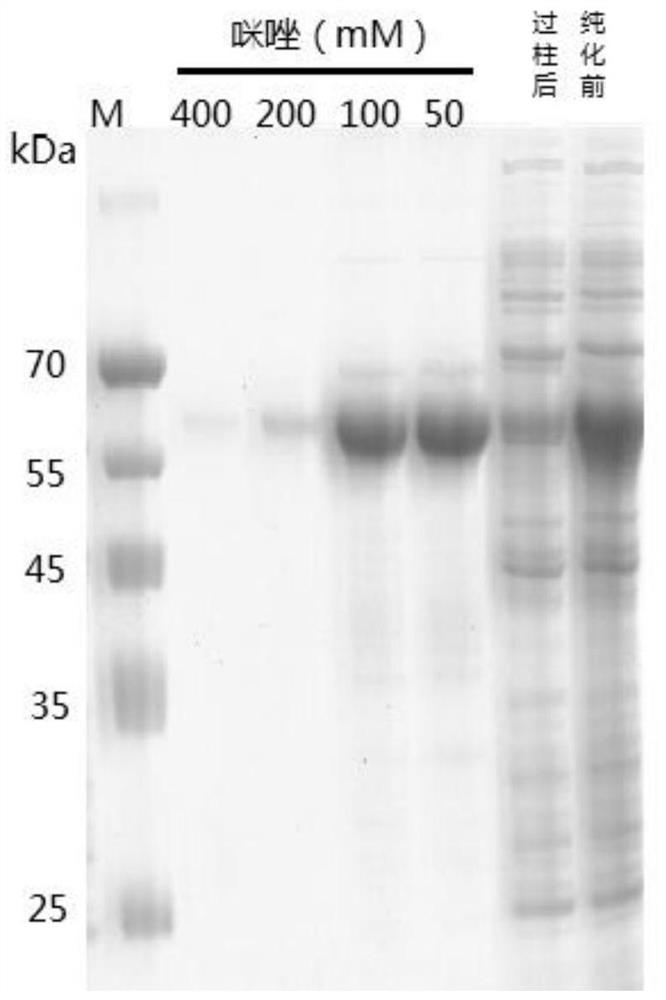
[0057] S2: Expression and extraction of soluble recombinant glycoside hydrolase family 7 protein: the Escherichia coli recombinant transformants containing the pET28 / GH7 vector of the optimized artificially synthesized gene were cultured in liquid LB medium at 37°C until OD 600 Then add IPTG with a concentration of 0, 0.1, and 0.5mM, respectively, and induce at 18°C for 24 hours. After the induction, the collected bacteria are ultrasonically cr...
Embodiment 3
[0066] (1) The present invention adopts the glucose hexokinase (HK) method to measure the ability of glycoside hydrolase family 7 proteolysis carboxymethylcellulose sodium (CMC-Na) to produce glucose, and hexokinase catalyzes glucose (D in the presence of ATP) -Glucose) to phosphorylate it to generate glucose-6 phosphate (G-6-P), G-6-P and coenzyme NAD generate NADH and 6-phosphogluconic acid under the action of glucose-6-phosphate dehydrogenase, which can The absorbance change of NADH at 340nm wavelength was measured by spectrophotometry, and the concentration of glucose in the sample was quantitatively detected. The specific steps and results were as follows: 2 μl of purified Trx-GH7 with a concentration of 1 mg / mL was added to 98 μl of 1% CMC- Sodium dihydrogen phosphate of Na and citric acid buffer solution (pH = 4), react at 40°C for 1 hour; take 10 μl and 2 μl of the reacted sample with a concentration of 10 mmol / L of the mixture of NAD and ATP, and add water to the total...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com