MntC recombinant protein of staphylococcus aureus and preparation method and application thereof
A technology of recombinant protein and Staphylococcus, which is applied in the biological field, can solve the problems of toxicity and achieve the effects of high expression, strong immunogenicity, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0095] Example 1: Codon optimization of encoding MntC and construction of expression vector and expression engineering bacteria
[0096] According to the coding preference of Escherichia coli, the coding of the gene SAR0641 encoding MntC in the MRSA-252 genome (GI: 49240382) was optimized as follows:
[0097] The 9th codon (encoding Leu) of the wild-type MntC protein coding sequence (SEQ ID NO.5) was optimized as CTG, the 13th and 14th codons (encoding Thr) were optimized as ACC, and the 27th, 65th, and 282nd codons The codon (encoding Gly) was optimized to GGT, the 214th codon (encoding Arg) was optimized to CGT, and the optimized nucleotide sequence (SEQ ID NO.2) was obtained. The amino acid sequence encoded by this sequence is shown in SEQ ID NO. The 6th to 290th bits of 1 are displayed.
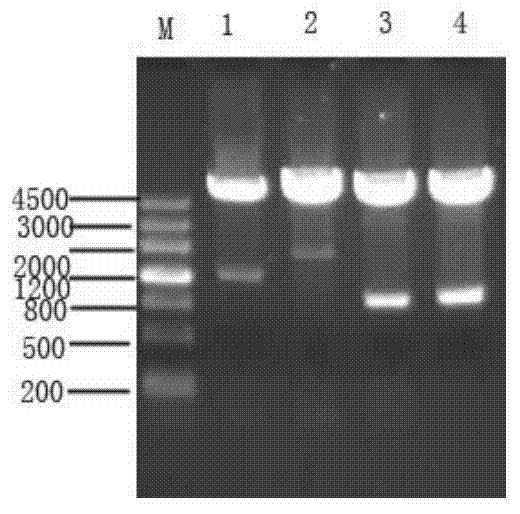
[0098] The optimized sequence was handed over to Shanghai Jierui Biotechnology Co., Ltd. for whole gene synthesis, and BamHI and NotI were used as the end-to-end restriction sites to lig...
Embodiment 2
[0099] Embodiment 2: the construction of wild MntC expression vector and expression bacteria
[0100] 1. Take the MRSA-252 strain and spread it on MH agar plate (Mueller-Hinton Agar, Beijing Aoboxing Biotechnology Co., Ltd., LOT: 02-051) for recovery, and cultivate it aerobically at 37°C overnight. Pick a single colony and inoculate 1000ml of MH (Mueller-Hinton, Beijing Aoboxing Biotechnology Co., Ltd., LOT: 02-052) liquid medium, culture at 37°C with aerobic shaking at 210rpm for 7h, and extract the MRSA genome.
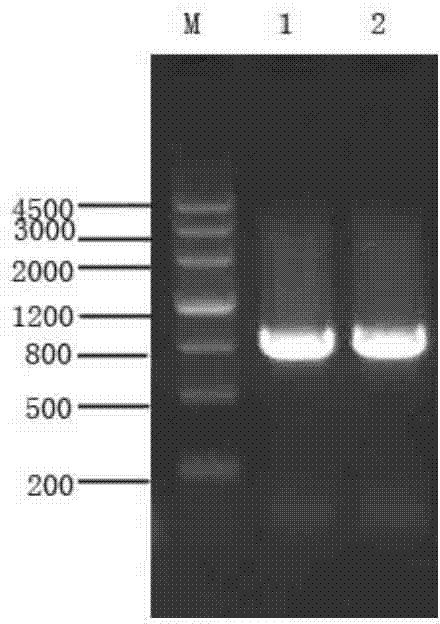
[0101] 2. Use the genome extracted in step 1 as a template to amplify the MntC target gene fragment. The amplification steps are as follows:
[0102] 1) Design the PCR primers as follows, respectively (the base sequence of the restriction site is underlined)
[0103] Forward primer: PMNTCBAMHI1 (SEQ ID NO.3:
[0104] 5'-CTG GGATCC AGCAGTGATAAGTCAAATGCAAAC-3';
[0105] BamHI
[0106] Reverse primer: PMNTCNOTI2 (SEQ ID NO.4):
[0107] 5'-AT GCGGCCGC CTTATTATT...
Embodiment 3
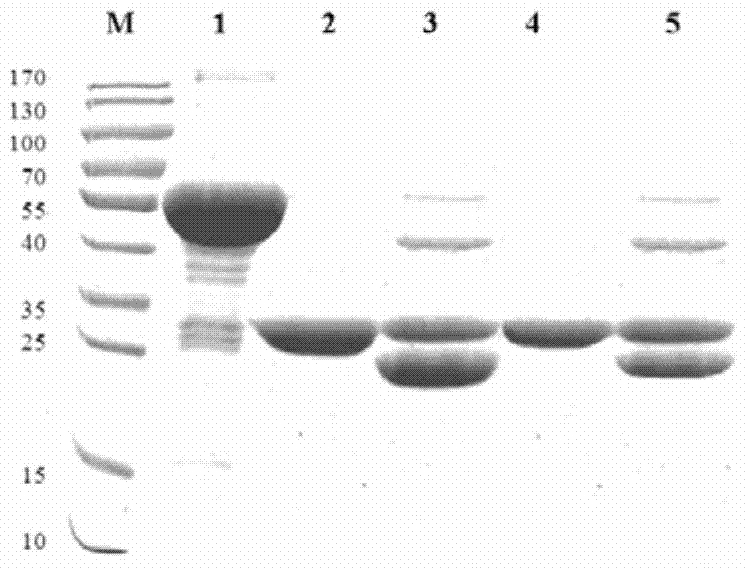
[0135] Example 3: Induced expression of MntC in prokaryotic expression system-Escherichia coli
[0136] 1. Induced expression of target protein
[0137] 1) Take 100 μL of the correctly identified pGEX-6P-2-MntC(wt) / XL1-blue bacterial solution and the whole gene synthetic pGEX-6P-2-MntC(r) / XL1-blue bacterial solution and add to 10mL containing 100μg / In the LB medium of ml Amp, cultivate overnight at 80rpm at 37°C, take 2mL of the overnight cultured bacterial solution and add them to 100mL LB medium containing 100μg / ml Amp; when the OD600 is 0.8-1.0, add IPTG to make the final concentration 200 μM, then placed on a shaking table to induce expression at 30°C for 3h, and then induced expression at 16°C overnight.
[0138] 2) Take out the bacterial solution after induced expression, centrifuge at 10000rpm for 5min, discard the supernatant, add 5mL of lysis buffer (PBS) to mix, and lyse with ultrasonic (power 300 watts) for 10min (work for 6 seconds, rest for 9 seconds), and then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com