Polyepitope tuberculosis gene vaccine and its prepn process
A gene vaccine and multi-epitope technology, applied in the direction of bacterial antigen components, antibacterial drugs, etc., can solve the problem of low level of vaccine-induced cellular immune response, and achieve the effect of enhancing the level of immune response against Mycobacterium tuberculosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
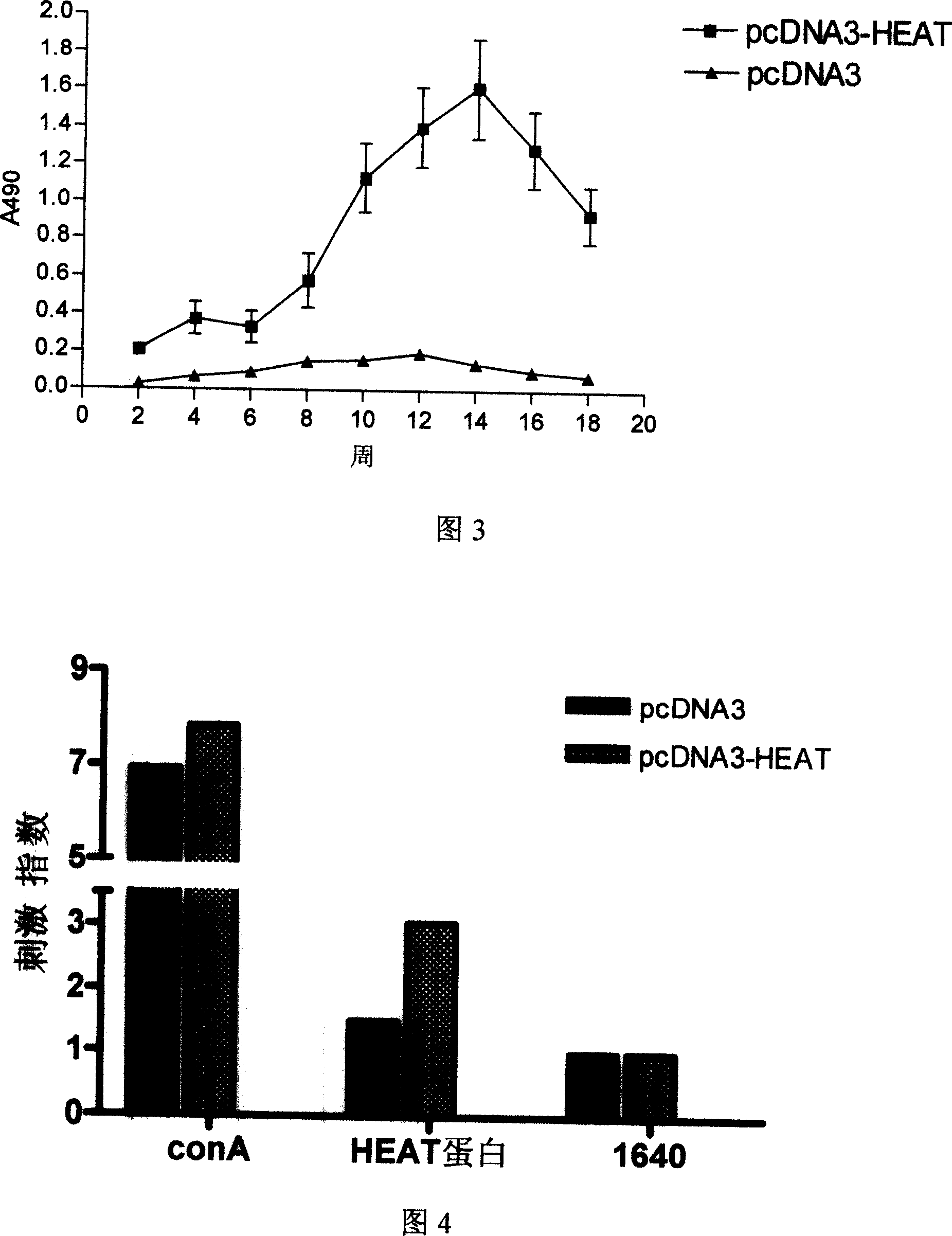
[0032] Immunological reagents and materials: goat anti-mouse CXCR3 polyclonal antibody (Sant Clous company); FITC-labeled donkey anti-goat IgG (ELISA titer 1:5000, Huamei Bioengineering Co., Ltd.); 2ml and 1ml sterile syringes (Mesha Wa Medical Industry Co., Ltd.). Embodiment 1: Design, construction, identification and expression of HEAT gene vaccine
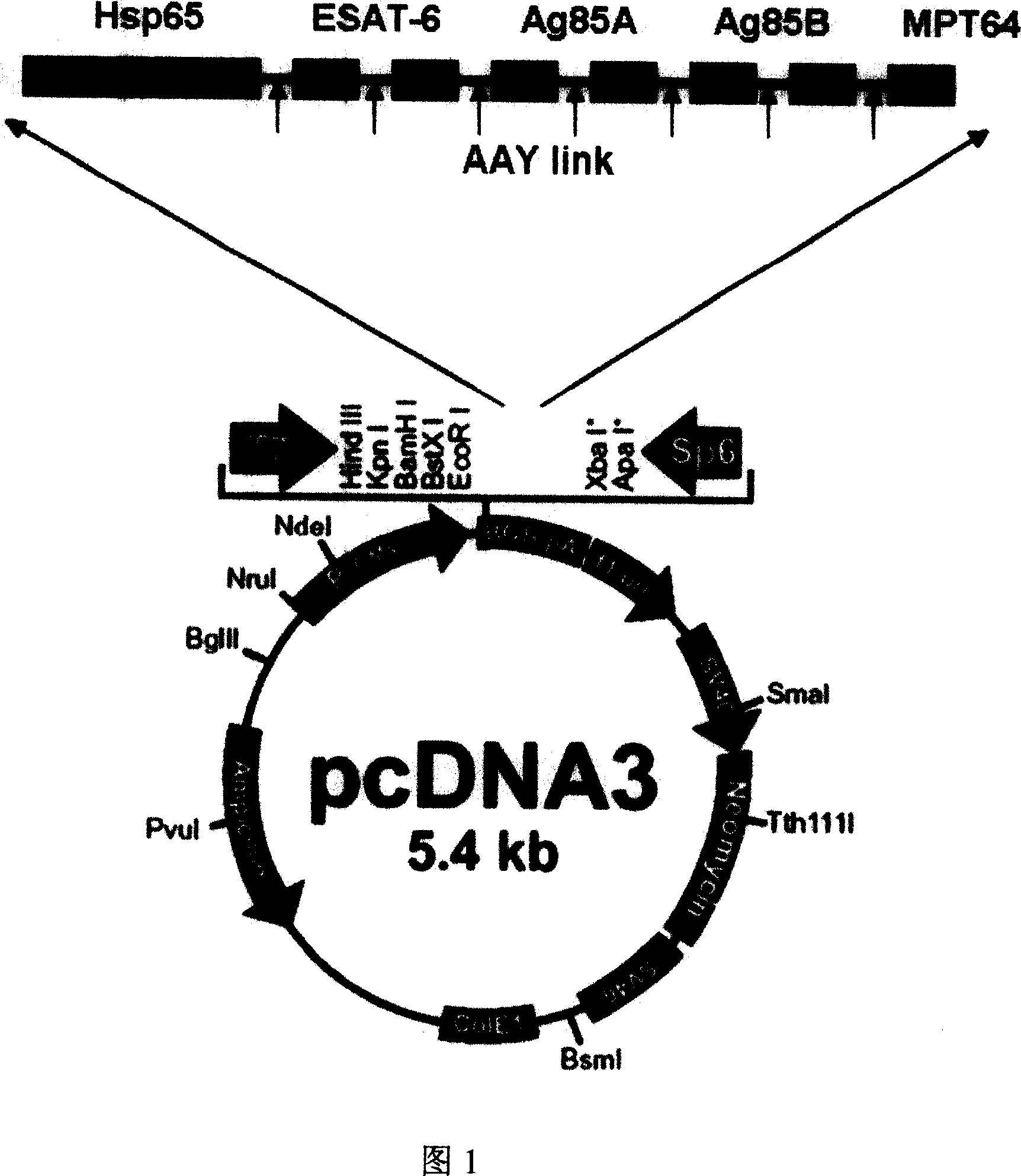
[0033] Using molecular biology software and network database prediction methods, combined with the relevant reports of the existing tuberculosis protein epitope research, it was determined that the full-length gene of Mycobacterium tuberculosis HSP65, the 1-20 gene and the 61-81 position of ESAT-6 Gene, Ag85A 62-84 gene, Ag85B 121-155 gene, Ag85A 143-166 gene, Ag85B 234-256 gene and MPT64C terminal 177-228 gene are connected in series to construct the gene vaccine of the present invention.
[0034]The present invention utilizes the PCR method and the direct DNA sequence synthesis method to amplify the target gene, and finally a...
Embodiment 2
[0101] Example 2 Construction of multi-epitope tuberculosis gene vaccine
[0102] Using the extracted Mycobacterium tuberculosis H37Rv strain (provided by the Tuberculosis Department of Shanghai Center for Disease Control and Prevention) DNA as a template, use HSP65 upstream (SEQ ID NO: 19) and downstream (SEQ ID NO: 20) specific primers by PCR Methods The HSP65 fragment was amplified, and the PCR product was recovered and purified by low melting point gel.
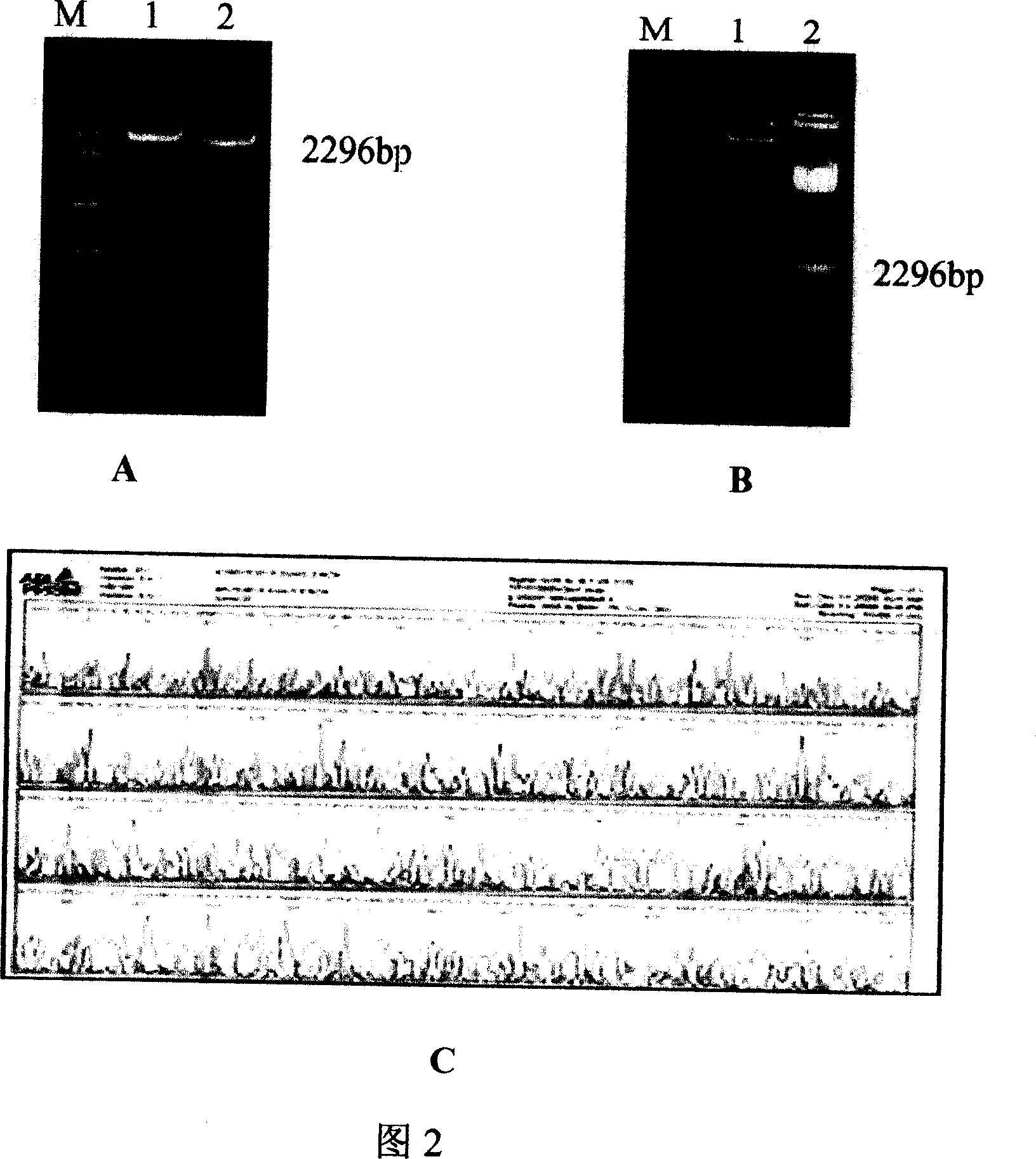
[0103] The designed cohesive-end complementary DNA sequence 1-7, with T 4 DNA ligase was ligated, and the ligated products were ligated with HSP65 and MPT64 amplified by PCR respectively; using the ligated products as templates, specific primers for HSP65 upstream (SEQ ID NO: 19) and MPT64 downstream (SEQ ID NO: 29) were passed through The PCR method amplifies the full-length gene fragment of the multi-epitope gene vaccine. The results are shown in Figure 2, and a full-length coding gene of 2296 bp was obtained, which ...
Embodiment 3
[0110] Example 3 Construction of pcDNA3-HEAT eukaryotic expression vector and plasmid purification
[0111] The amplified HEAT coding gene fragment was double digested with EcoR I and Xba I, and the digested fragment was recovered, ligated with the corresponding digested vector pcDNA3, the ligated product was transformed into Escherichia coli DH5α, and the transformed colonies were screened with ampicillin. After identification by PCR, enzyme digestion and sequencing, the pcDNA3-HEAT eukaryotic expression plasmid was successfully constructed (Fig. 2A, 2B, 2C).
[0112] The recombinant plasmid pcDNA3-HEAT was transformed into Escherichia coli DH5α, positive clones were screened, cultured with shaking in LB (Amp100μg / ml) liquid medium for 15 hours, the bacteria were collected, and impurities and bacterial endotoxins were removed according to the QIAGEN Plasmid Mega Kit, and finally purified Plasmid DNA, our gene vaccine pcDNA3-HEAT.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com