Antigen protein specifically bound with tyrosine phosphatase antibody
A tyrosine phosphatase, specific binding technology, applied in the field of antigen-antibody protein, truncated tyrosine phosphatase, can solve low efficiency, insufficient specificity, long, at least 6 hours, or even overnight reactions, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
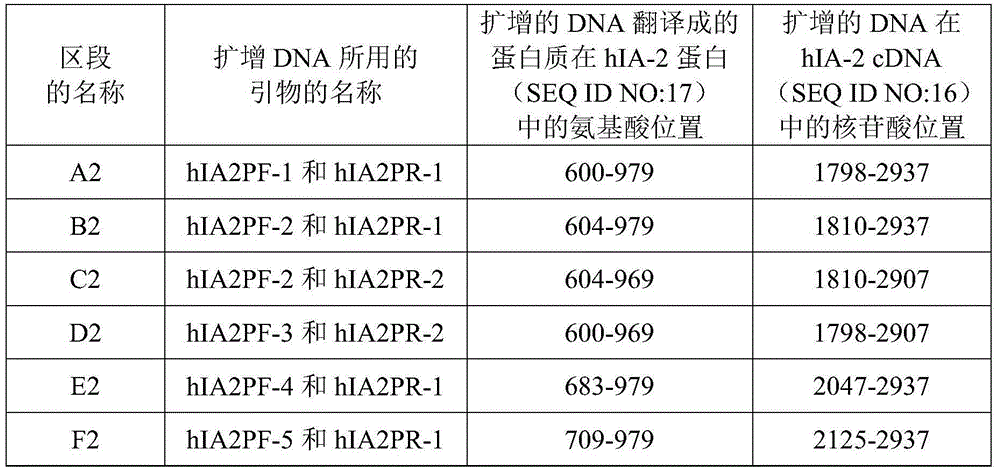
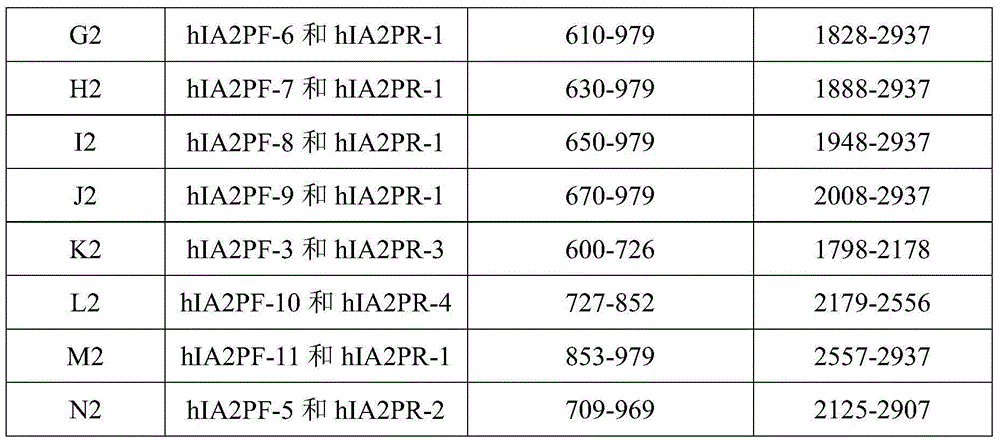
[0030] Expression of hIA-2 and truncated hIA-2 genes
[0031] (1) Main reagents and equipment
[0032] PCR kits (including Taq enzyme, buffer, dNTP, T4 ligase) and DNA markers were purchased from full gold; protein markers were purchased from Beyond Biotech; BamH I, Xho I, EcoR I, Nco I, HindⅢ and other restriction internal Dicer was purchased from Takara (Dalian Bao Biology); calf intestinal alkaline phosphatase (CIAP) kit (including components: calf intestinal alkaline phosphatase, alkaline phosphatase buffer) was purchased from Takara; gel recovery reagent Kits and plasmid extraction kits were purchased from OMEGA; kanamycin and ampicillin were purchased from BBI; IPTG was purchased from BBI; PCR primers and recombinant plasmid sequencing were completed by BGI or Invitrogen; other reagents were provided by Shenzhen New Industry Biomedical Engineering Co., Ltd. Inc. provided.
[0033] The ABI Veriti PCR instrument was purchased from ABI, the horizontal electrophoresis i...
Embodiment 2
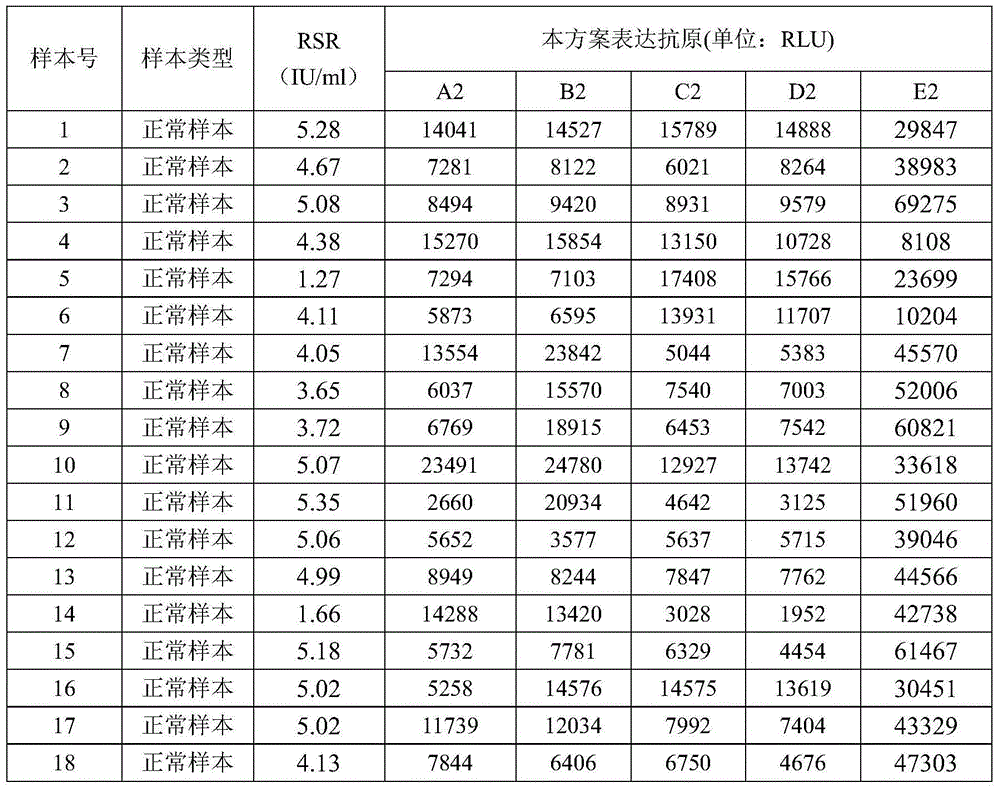
[0046] Determination of the sensitivity and specificity of the 14 antigens expressed by the above insect cells to the hIA-2 antibody
[0047] The principle of the chemiluminescent immune sandwich method: the serum sample to be tested, the buffer solution and the antigen-coated magnetic microspheres are added to the reaction cup for reaction, and at 37°C, the antibody in the serum sample to be tested and the antigen coated on the magnetic microspheres are immune In the case of a magnetic field, the nano-magnetic beads will be magnetized rapidly, and the complex will be washed to obtain the complex of the antigen and the antibody to be tested. Other components will be washed away, and then the labeled protein A-NHS-ABEI will be added. Protein A can Quickly and specifically bind to the C-terminus of the antibody to be tested to form a "sandwich" immune complex. When the detected antibody content in the serum sample is more, the relative light intensity RLU detected by the instrum...
Embodiment 3
[0068] Comparison of prokaryotic and eukaryotic expressed proteins for detection of type 1 diabetes
[0069] The truncated protein N2 of hIA-2 was expressed in Escherichia coli, and the truncated DNA segment of hIA-2 was connected to the pGEX4T-1 expression vector, and the expression strain of Escherichia coli was BL21(DE3). After the expression product is purified by GST tag, the GST tag is cut off with Thrombin, and the untagged target protein is obtained by affinity chromatography.
[0070] The sensitivity and specificity of prokaryotic expressed N2 to hIA-2 antibody were determined.
[0071] The symbol for the prokaryotic expression segment of hIA-2 truncated form is PN2, which corresponds to N2 for eukaryotic expression.
[0072] The detection platform and scheme details are the same as in Example 1, and the results are shown in Table 4.
[0073] Table 4
[0074]
[0075] The results of PN2 in Table 4 are compared with the results of N2. The non-specific binding of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com