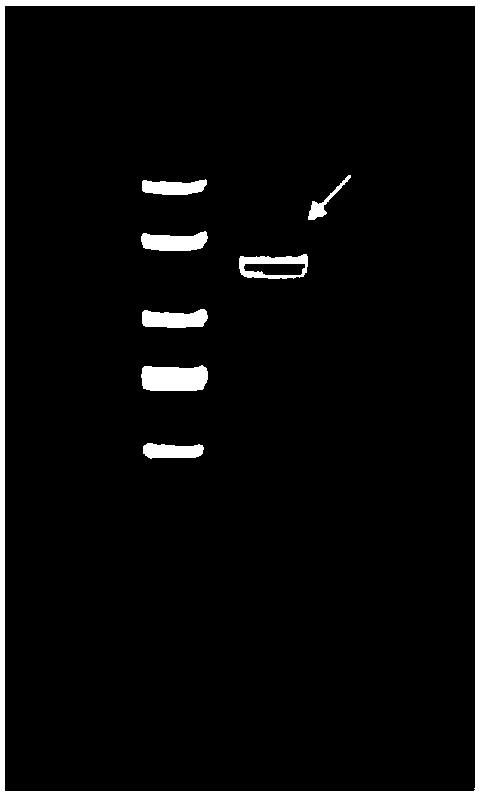
Duck tembusu virus E truncated protein and application
A duck Tembusu virus and truncated protein technology, which is applied in the direction of anti-viral immunoglobulins, viruses, and viral peptides, can solve the problem of unstable expression, and achieve high sensitivity, large sample size, and large expression Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Obtaining of E truncated protein of DTMUV DF2 strain:
[0050] (1) DTMUVDF2 chicken embryo virus, take 200 μL, add 800 μL of Trizol reagent, mix well, let stand at room temperature for 5 minutes, add 200 μL of chloroform, shake vigorously for 15 seconds, ice bath for 10 minutes, 4 ° C, 12000 rpm, centrifuge for 15 minutes, take 600 μL Put the supernatant into a clean EP tube, add an equal volume of isopropanol solution, mix gently, let stand at room temperature for 10min, 4°C, 12000rpm, centrifuge for 10min, discard the supernatant, add 1mL absolute ethanol to the EP tube, mix gently , 4°C, 7500rpm, centrifuge for 5min, discard the supernatant, dry at room temperature, add 13μL DEPC water to dissolve, and store at -20°C.
[0051] (1) reverse transcription:
[0052] Add components according to the following system: AMV reverse transcriptase 0.5 μL, 5 times AMV reverse transcription buffer 4 μL, RRI 1 μL, random primer 1 μL, dNTPS 2 μL, RNA template 11.5 μL, a total of 2...
Embodiment 2
[0070] Obtaining of hybridoma cell line 4A10:
[0071] (1) Three healthy ducks were taken, blood was collected from the carotid artery, placed at 37°C for 30 minutes, and then separated at 4°C overnight, and the serum was collected. Extract duck serum IgG with caprylic acid-ammonium sulfate method, the specific operation is as follows: centrifuge the collected duck serum at 4°C and 4000rpm for 3 minutes, take the supernatant, add 4 times the volume of 60mM / L sodium acetate solution of pH 4.5, add as you go Stir, add 25 μL of n-octanoic acid per 1 mL of the above serum, let stand at room temperature for 30 min, centrifuge at 12,000 rpm for 10 min at 4°C, take the supernatant, filter with filter paper, and adjust the pH to 7.4 after filtration. Add a final volume of less than or equal to 45% saturated ammonium sulfate solution while stirring, stir at room temperature for 30 minutes, centrifuge at 4000rpm at 4°C for 10 minutes, remove the supernatant, resuspend the precipitate wi...
Embodiment 3
[0081] Obtaining enzyme-labeled secondary antibodies
[0082] (1) Preparation and purification of mouse anti-duck IgG mouse ascites:
[0083] The hybridoma cell line 4A10 was taken out from the liquid nitrogen tank, revived, and expanded for culture. Three days to one week in advance, BALB / C female mice aged 8-10 weeks were injected intraperitoneally with 0.5 mL of Freund's incomplete adjuvant per mouse. The expanded hybridoma cells were counted with a cell counting plate, according to 5*10 5 Each mouse was injected intraperitoneally, and after 7-10 days, the ascites of the mice was collected every day until the mice died. The collected ascites was centrifuged at 12000 rpm for 5 mins, the precipitate was discarded, thimerosal was added to the supernatant with a final concentration of 0.01%, and stored at -80°C after aliquoting. Purify according to caprylic acid-ammonium sulfate method to obtain 4A10 monoclonal antibody, ie mouse anti-duck IgG.
[0084] (2) HRP-labeled mous...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com