Streptococcus suis truncated protein Sao and application thereof
A technology for Streptococcus suis and truncated proteins, which can be applied in applications, antibacterial drugs, and veterinary vaccines. It can solve the problems of difficult purification of full-length Sao protein, obstacles to the development of Sao protein vaccines, and poor protein stability. It is not easy to achieve Degradation, stable properties, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of Streptococcus suis truncated protein Sao and vaccine preparation:
[0031] 1.1 Synthesis of truncated protein Sao
[0032] The sequence of the truncated protein Sao is shown in SEQ ID NO.2, which was synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. The truncated protein Sao polypeptide after synthesis has a purity of 99%, 5 mg each, and is in the form of white powder. It is stored in the dark at -20 degrees and is completely soluble in water. The protein was dissolved in PBS buffer solution, stored at -80°C for one month, and no protein degradation was found after taking it out.
[0033] Before use, wash with sterile PBS (NaCl 8.0g, KCl 0.2g, KH 2 PO 4 0.24g, Na 2 HPO 4 ×12H 2 O 3.628g, pH7.4) was diluted according to the specified concentration. Preheat in a 37-degree incubator before use.
[0034] 1.2 Preparation of vaccine
[0035] Dosage form: water-in-oil (W / O)
[0036] Adjuvant composition: white mineral oil (Marcol52), Tween 80 (C...
Embodiment 2
[0045] Application of Truncated Protein Vaccine Sao in Preparation of Streptococcus Suis Infection Vaccine
[0046] 1. Evaluate the immune protection effect of the vaccine on the mouse model
[0047] 1.1 Experimental scheme
[0048] Experimental animals: 4-week-old female BALB / c mice, purchased from Hubei Provincial Center for Disease Control and Prevention.
[0049] Challenge strain: Streptococcus suis type 2 SC19.
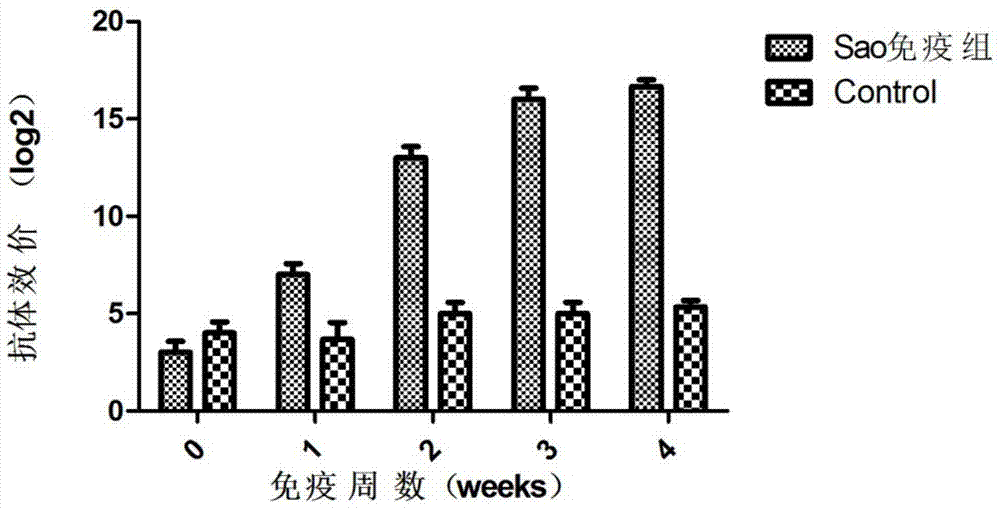
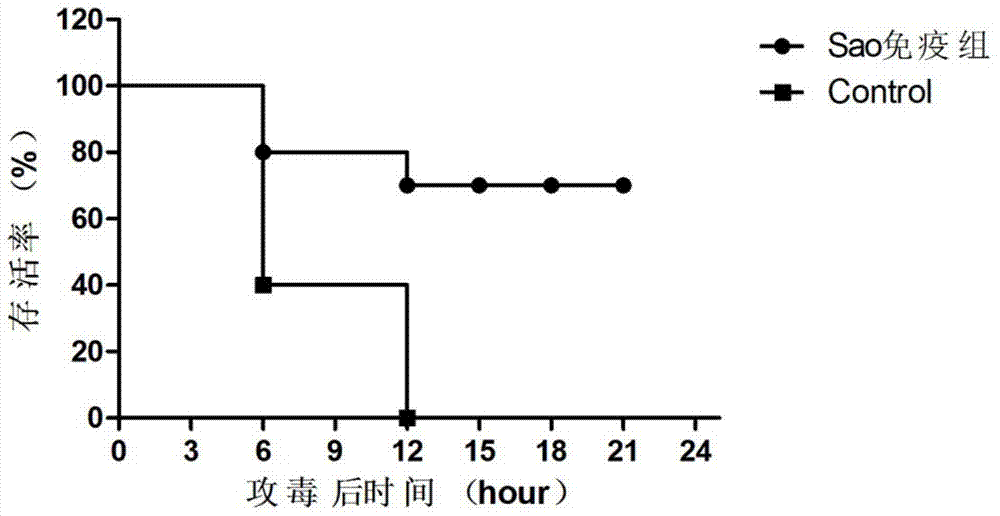
[0050] Experimental grouping: 20 female BALB / c mice weighing 16g were randomly divided into 2 groups: vaccine group I (Sao) and control group (adjuvant and PBS), 10 in each group. During the period after immunization, blood was collected by tail docking every week for the monitoring of serum-specific antibodies. Immunization method: subcutaneously immunize experimental mice with the vaccine prepared in Example 1 at a dose of 100 μl / only; booster immunization once 2 weeks later (immunization dose and site are the same as the first immunization).
[0051] 1.2 D...
Embodiment 3
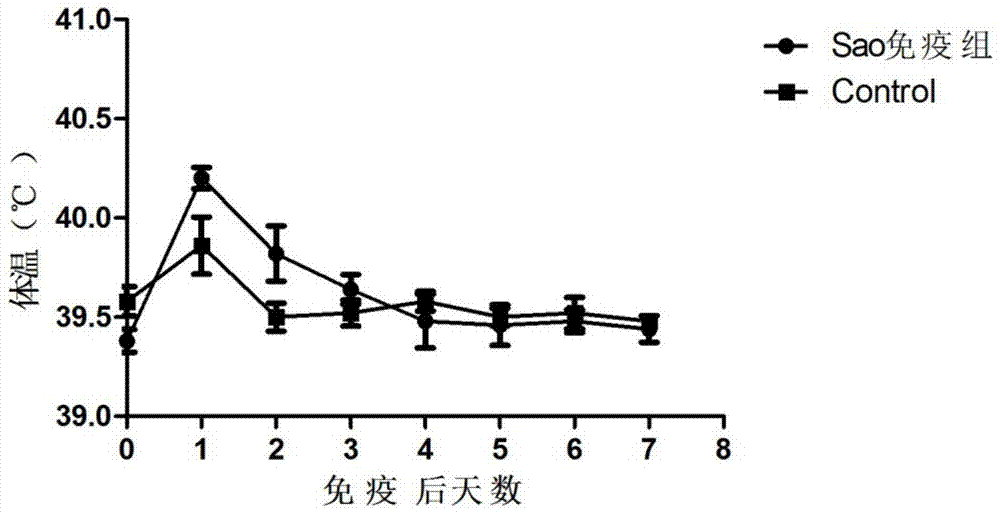
[0074] Embodiment 3: Safety inspection
[0075] In order to test the safety impact of the prepared vaccine on piglets and pregnant sows, the safety test was carried out on 35-day-old piglets and 70-day-old sows.
[0076] 3.1 Experimental scheme:
[0077] 1) Inoculate 35-day-old healthy piglets with the prepared vaccine by point injection through the buttock muscle, inject 5 piglets in each batch of vaccine, and inoculate 2ml into each piglet, observe their clinical manifestations and measure their body temperature.
[0078] 2) The prepared vaccine was inoculated into healthy sows on the 70th day of gestation through hip muscle injection respectively, and each batch of vaccine was injected into 5 pigs, and each pig was inoculated with 2ml, and the clinical manifestations and litter conditions were observed.
[0079] 2. Test results
[0080] 3.2.1 The safety of the subunit vaccine of the present invention to the vaccination of weaned piglets
[0081] After the prepared vaccin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com