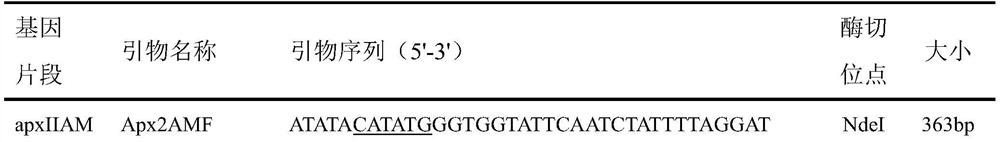Porcine infectious actinobacillus pleuropneumoniae subunit vaccine
A subunit vaccine, pleuropneumonia technology, applied in vaccines, veterinary vaccines, bacterial peptides, etc., can solve the problem of multi-component rApxIIIAN incapacity, and achieve the effect of low overall cost and easy production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] (1) Construction of recombinant expression plasmids
[0016] Using the genomic DNA of Actinobacillus pleuropneumoniae as a template, the target fragment was amplified by PCR using the primers shown in Table 1.
[0017] Table 1 Primer information
[0018]
[0019]
[0020] The amplified fragments of interest and the plasmid pET-20b(+) were digested with NdeI and XhoI respectively, and then ligated and transformed into Escherichia coli DH5α to finally construct the recombinant expression plasmids pET20b-apxIIAM, pET20b-omlA and pET20b -tbpB. These recombinant expression plasmids were identified by enzyme digestion and sequencing, all of which were in line with expectations, without any mutation in the sequence, and successfully inserted into the pET20b vector.
[0021] (2) Expression and purification of recombinant protein
[0022] The constructed recombinant expression plasmids pET20b-apxIIAM, pET20b-omlA and pET20b-tbpB were transformed into Escherichia coli BL...
Embodiment 2
[0025] The concentrations of the purified recombinant proteins in Example 1 were measured by the BCA method, and the recombinant proteins were combined in a certain proportion and then mixed with aluminum hydroxide adjuvant in equal volumes to obtain three components with a content of each recombinant protein of 200 μg / mL Recombinant subunit vaccines are used in the following immune challenge experiments.
[0026] Vaccine sterility test: Aseptically draw 200 μL of the prepared vaccine into a plate coated with TSA+NAD (nicotinamide adenine dinucleoside, 5 μg / mL), culture at 37° C. overnight, no colonies grow on the plate.
Embodiment 3
[0028] The safety and effectiveness of the three-component recombinant subunit vaccine obtained in Example 2 were verified through piglet clinical trials, and the piglets were protected by the Actinobacillus pleuropneumoniae subunit inactivated vaccine of Merck & Co. Epco APP) for comparison. Before the pigs were immunized, the blood was collected and the serum was tested with the Actinobacillus pleuropneumoniae differential diagnosis kit to ensure that the test pigs were all negative pigs.
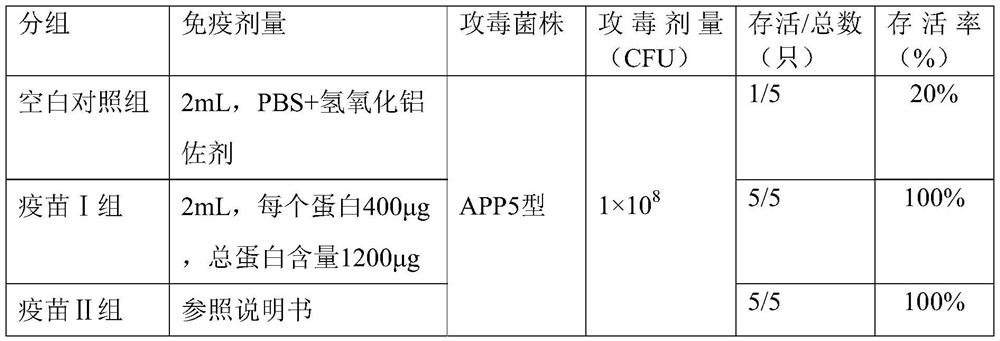
[0029] The 8-week-old piglets were divided into 3 groups for immunization, with 5 piglets in each group, and the experimental groups were as follows:
[0030] Vaccine group I: rApxIIAM, rOmlA, rTbpB three-component recombinant subunit vaccine;
[0031] Vaccine group II: MSD Actinobacillus pleuropneumoniae subunit inactivated vaccine (containing 1 outer membrane protein and 3 toxins ApxI, ApxII, ApxIII);
[0032] Blank control group: PBS + aluminum hydroxide adjuvant.
[0033] Immune...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com