Broadly protective inactivated influenza virus vaccine
A technology of influenza virus and influenza A virus, applied in antiviral agents, virus/bacteriophage, antibody medical components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0162] Example 1: Chemically Inactivated Influenza Virus Mixture as a Universal Influenza Vaccine
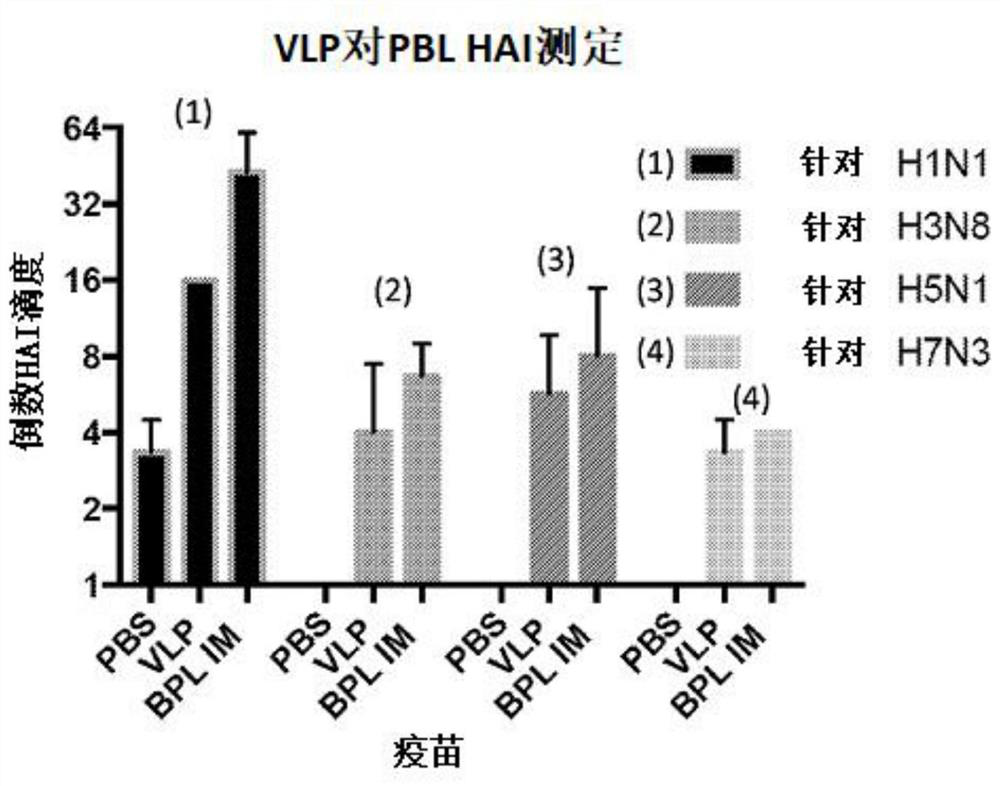
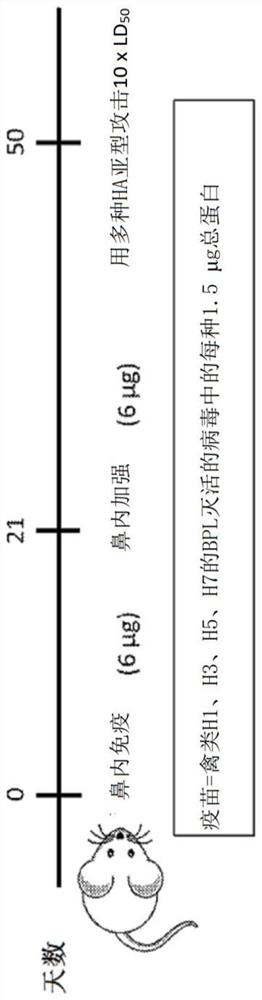
[0163] This example describes a study evaluating an influenza virus vaccine consisting of a mixture of BPL-inactivated low pathogenicity avian influenza virus subtypes. The first vaccine mixture includes influenza viruses with HA subtypes H1, H3, H5, and H7, and the second vaccine mixture includes influenza viruses with HA subtypes H2, H4, H9, and H10 (see image 3 ). The influenza virus used in the assay is monovalent (eg, only includes a single or separate subtype of HA.
[0164] A quadrivalent vaccine (A / mallard / Ohio / 265 / 1987(H1N9), A / pintail / Ohio / 339 / 1987(H3N8), A / mallard / Maryland / 802 / 2007 (H5N1) and A / Environment / Maryland / 261 / 2006 (H7N3)) were compared with virus-like particle (VLP) vaccines comprising VLPs expressing HA from the same virus. figure 1Reciprocal HAI titers of mice vaccinated with quadrivalent influenza VLP vaccine or with BPL-inactivated whole virus quadr...
Embodiment 2
[0170] Example 2: Testing of Inactivated Influenza Virus Vaccines in Ferrets
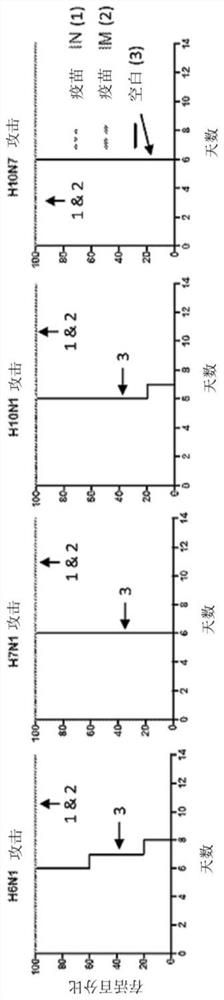
[0171] Not infected by influenza (influenza ) Purebred ferrets (Mustela putorius furo, female, 6-12 months old) were purchased from Marshall Farms (Sayre, PA, USA). Ferrets were housed in pairs in stainless steel cages (Shor-line, Kansas City, KS, USA) including Sani-chips Laboratory Animal Bedding (P.J. Murphy Forest Products, Montville, NJ, USA). ). Ferrets were provided the Teklad Global Ferret Diet (Harlan Teklad, Madison, WI, USA) and had water ad libitum.
[0172] Ferrets were blank vaccinated or IN or IM with a quadrivalent vaccine consisting of 4 different BPL-inactivated whole influenza viruses: A / mallard / Ohio / 265 / 1987(H1N9), A / pintail / Ohio / 339 / 1987 (H3N8), A / mallard / Maryland / 802 / 2007 (H5N1), and A / Environment / Maryland / 261 / 2006 (H7N3). Two ferrets were included in each vaccination group. Ferrets were challenged with antigenic variant and pathogenic strains A / Port Chalmers / 1971 (H3N2)...
Embodiment 3
[0178] Example 3: Human Clinical Trials
[0179] After selecting the best broadly cross-reactive inactivated influenza virus vaccine in laboratory animals, it was studied in human volunteers. In some instances, the vaccine will include an adjuvant suitable for human use.
[0180] Will be produced using GMP methods (e.g. tetravalent H1 / H3 / H5 / H7, tetravalent H2 / H4 / H9 / H10 or octavalent H1 / H3 / H5 / H7 / H2 / H4 / H9 / H10) including inactivation (e.g. BPL-inactivated) whole influenza vaccine formulation and administered intranasally to humans. Those of skill in the art will appreciate that other inactivated influenza virus vaccine compositions provided herein can be similarly tested.
[0181] Briefly, humans were vaccinated intranasally with influenza vaccine compositions. The human is boosted with the same mixture about 3-12 weeks later (eg, 3, 4, 5, 6, 7, 8, 9, 10, 11 or 12 weeks later). A second group of people is blank vaccinated (eg, with saline). Obtain and store blood and nasal s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com