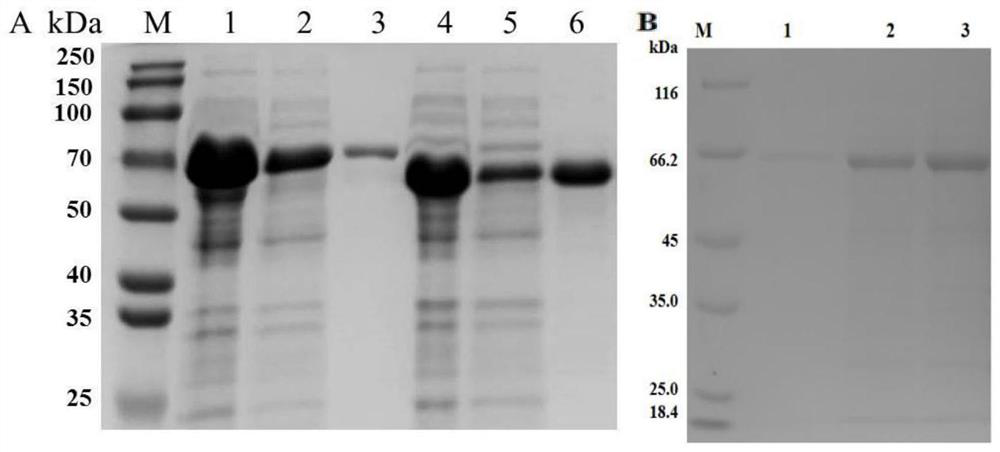
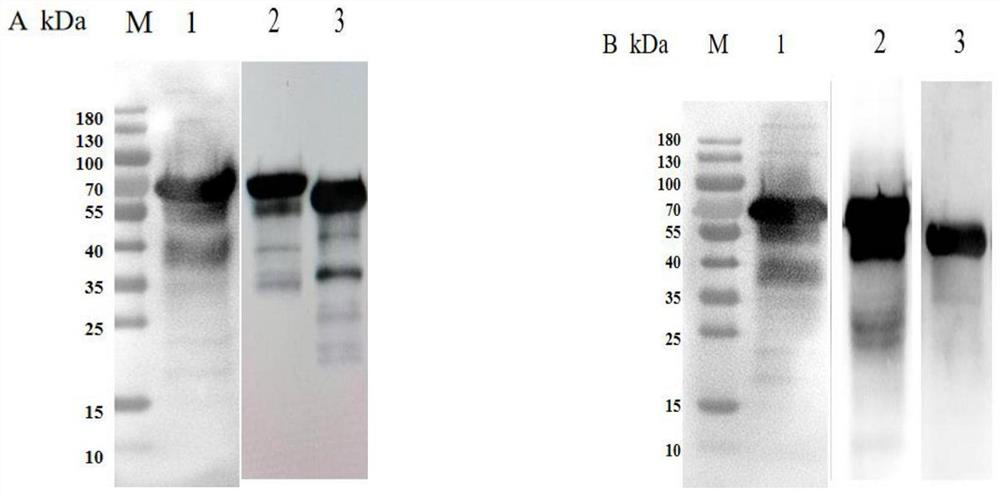
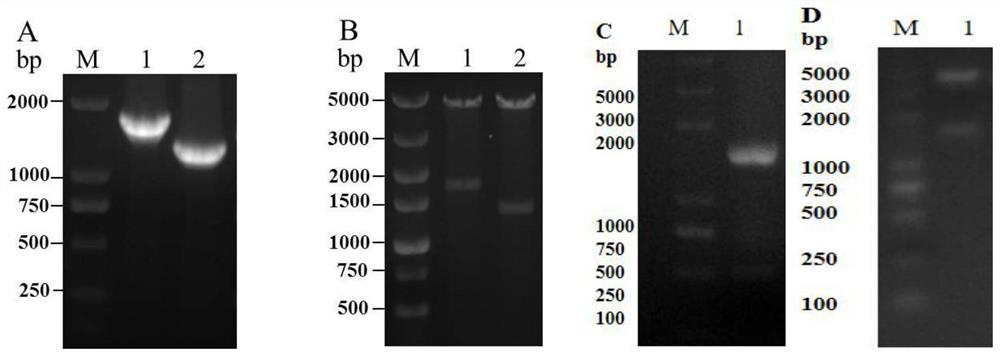
Actinobacillus pleuropneumoniae subunit vaccine
A technology of subunit vaccines and actinobacillus, which is applied in the direction of vaccines, veterinary vaccines, bacterial peptides, etc., to achieve the effect of low overall cost and easy production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0030] The preferred embodiments of the present invention will be described below in conjunction with the accompanying drawings. It should be understood that the preferred embodiments described here are only used to illustrate and explain the present invention, and are not intended to limit the present invention.
[0031] 1 Material method
[0032] 1.1 Experimental animals
[0033] Sixty-four 4-week-old specific pathogen-free (SPF grade) ICR mice were purchased from Shanghai Xipuer-Bicai Laboratory Animal Co., Ltd.
[0034]1.2 Main reagents
[0035] IgG1 and IgG2a antibodies were purchased from Nanjing Tianwei Company; reverse transcriptase was purchased from Novozyme; restriction endonucleases Sac I, Xho I, Hind III, and protein molecular mass standards were purchased from Thermo Fisher Scientific (China) Company; Mouse Peripheral Blood Lymphocyte Separation Liquid KIT was purchased from Tianjin Haoyang Biological Products Technology Co., Ltd.; Carbomer 971P and ISA-15A wer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com