West nile virus monoclonal antibody and kit
A West Nile virus and monoclonal antibody technology, applied in the direction of antiviral immunoglobulin, biochemical equipment and methods, instruments, etc., can solve the problems of easy contamination of samples and operations, expensive consumables, false positives, etc. Achieve the effect of simple operation, rapid response and strong sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1, West Nile virus monoclonal antibody hybridoma cell, the preparation of monoclonal antibody and polyclonal antibody
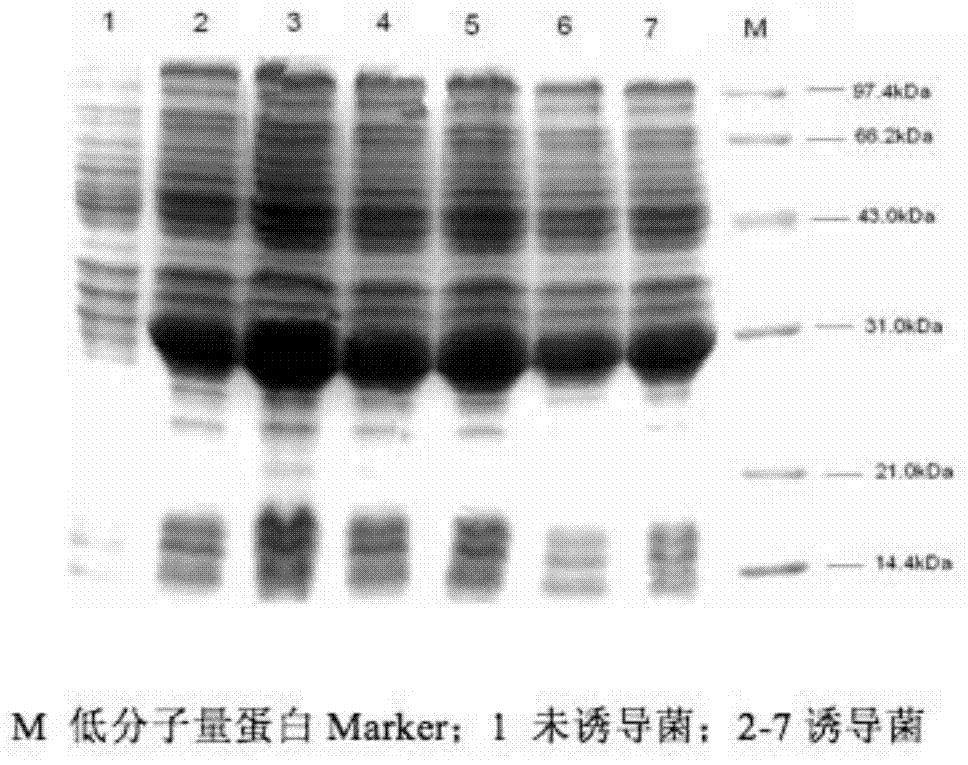
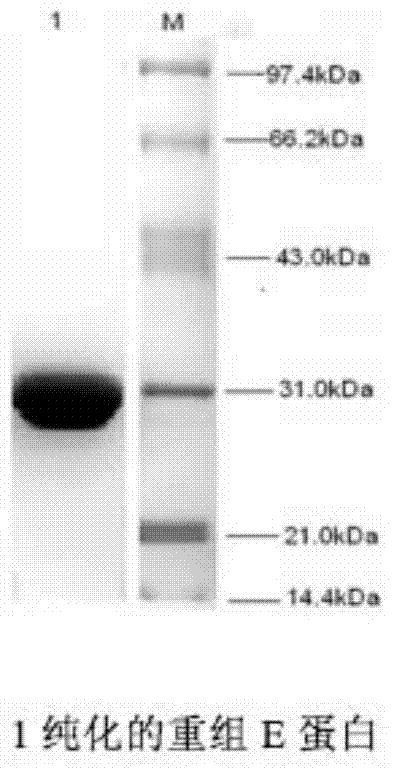
[0042] 1. Recombinant expression and purification of West Nile virus (WNV) envelope E protein.
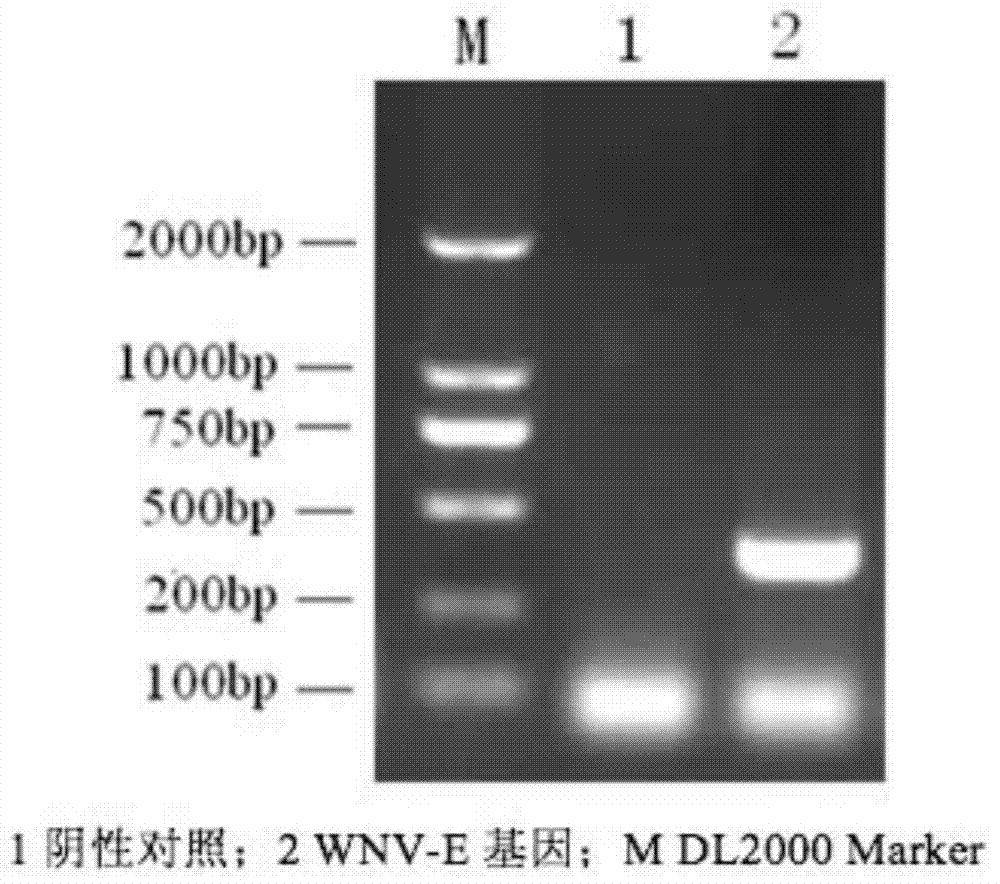
[0043] In order to express the recombinant WNV E protein, the nucleic acid sequence of domain III of the E protein (the nucleic acid sequence encoding the 296-415 amino acids of the E protein) was artificially synthesized. Primers were designed according to the West Nile virus E protein gene. The upstream primer was 5'-AGCTCGAGCATGCAGTTGAAGGGAACAACCTATG-3', and the Xho I restriction site was introduced at the same time. The downstream primer was: 5'-TTTGGATCCTTACGCTCCTTTGAGGGTGG-3', and the BamHI restriction site was introduced. The target gene was cloned using the artificially synthesized WNV-E gene sequence as a template. PCR amplification program: 94°C for 4min, 94°C for 30s, 50°C for 30s, 72°C for 30s, 35 cycles, 72°C for 10min. The amplifi...
Embodiment 2
[0058] Embodiment 2: Titer determination of West Nile virus monoclonal antibody ascitic fluid
[0059] 1. Use the indirect ELISA method to measure the ascites titer of West Nile virus monoclonal antibody.
[0060] The specific method is:
[0061] 1) Coat the microtiter plate with purified West Nile virus E protein at a concentration of 1 μg / mL, freeze overnight at 4°C, and wash the microtiter plate.
[0062] 2) Seal the plate with phosphate buffer saline containing 2.5% BSA, wash the microplate at 37° C. for 30 min.
[0063] 3) Add ascites antibody solution diluted according to a certain ratio, 37°C, 2h. Wash the microtiter plate.
[0064] 4) Add alkaline phosphatase-labeled goat anti-mouse antibody diluted 1:1000, 37°C, 2h. Wash the microtiter plate.
[0065] 5) Add the substrate solution, at 37°C, read the OD value at 450nm with a microplate reader when the color is suitable (5-15min).
[0066] Such as Figure 4 As shown, the ascites antibody titer of Example 1 of the...
Embodiment 3
[0067] Embodiment 3: Ascites specificity determination of West Nile virus monoclonal antibody
[0068] 1. Identification of immunoglobulin classes and subclasses of monoclonal antibodies
[0069] 1. Follow the operating instructions of the Southern Biotech kit
[0070] (1) Coating: Dilute the antibody provided in the kit with carbonate buffer solution of pH 9.6 to a concentration of 5-10 μg / Ml100 μ / well, overnight at 4°C;
[0071] (2) Washing: wash the plate 3 times with PBS containing 0.05% Tween-20, and pat dry;
[0072] (3) Blocking: add 200 μL / well of PBST containing 1% bovine serum albumin, and incubate at 37° C. for 1 h to block the uncoated active site; wash as above;
[0073] (4) Sample addition: add 100 μL / well of the hybridoma culture supernatant of Example 1, place on a microtiter plate and incubate at 37° C. for 1 h; wash as above;
[0074] (5) Enzyme addition: Dilute alkaline phosphatase-labeled immunoglobulins, subclasses, heavy chains and light chains with PB...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com