African swine fever virus wild virus infection and gene deletion virus strain dual fluorescence quantitative PCR detection composition, method and kit
An African swine fever virus and detection kit technology, applied in the biological field, can solve the problems affecting the early differential diagnosis of African swine fever virus infection, inability to distinguish between the positive of wild virus infection and the positive of vaccine immunity, and achieves good social application prospects, good Consistent, well-specific results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Design and synthesis of embodiment 1 primers and probes
[0044] According to the published ASFV genome sequence in NCBI GenBank data, the B646L and MGF505-2R gene sequences of the popular strain Pig / HLJ / 2018 (Accession no.MK333180) in my country were selected (Table 1), and Primer3Plus (http: / / www. primer3plus.com) software for primer and probe design. Primers and probes were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. Primers and probes were diluted to 10 μmol / L with sterile water and stored at -20°C.
[0045] Table 1 Primer and Probe Information
[0046]
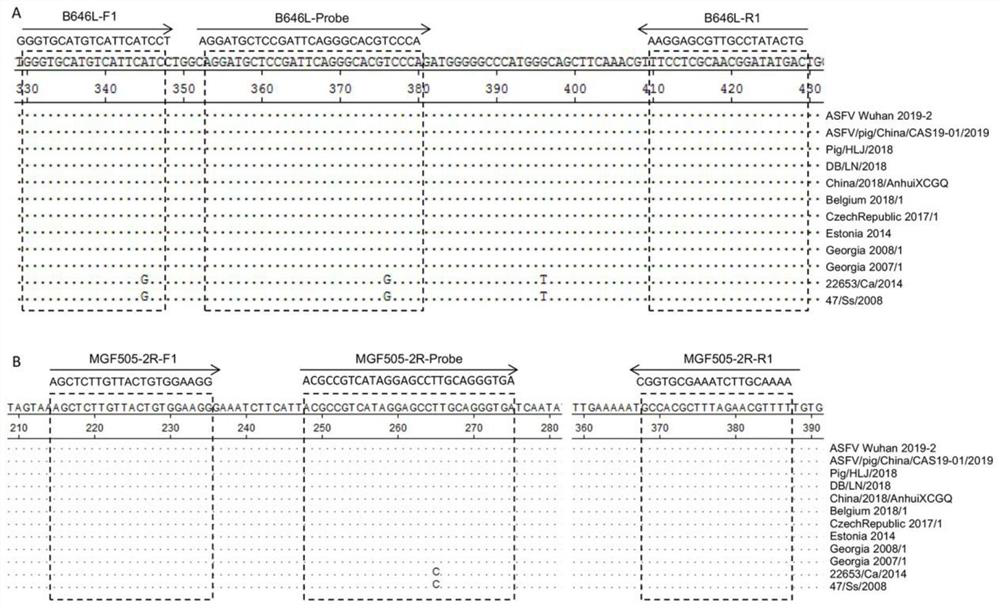
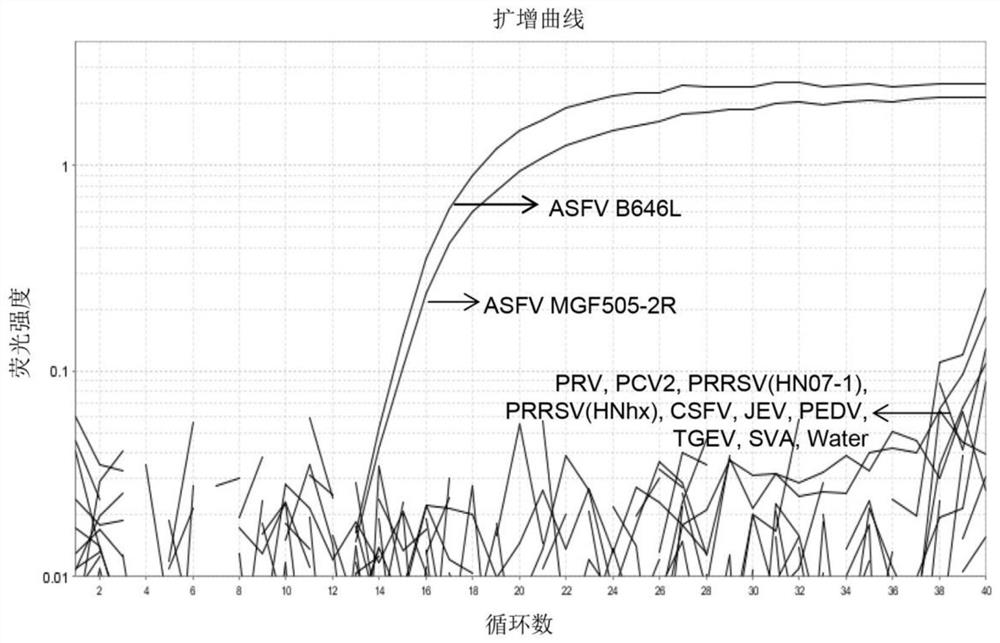
[0047] Further use the DNAstar Megalign software to evaluate the sequence conservation of the primers and probes in the prevailing strains, the selected strains are shown in Table 2, and the results are shown in figure 1 . Depend on figure 1 B646L and MGF505-2R gene sequence comparison analysis shows that the primers and probe sequences designed by the present invention are highly conserved amon...
Embodiment 2
[0050] The selection of positive control in embodiment 2 kit
[0051] The standard plasmids pUC57-B646L and pUC57-MGF505-2R were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. and kept in our laboratory. The standard plasmid concentration was determined by NanoDrop One (Thermo Fisher Scientific). The gene copy number calculation formula is as follows: Y (copies / μL) = [concentration of plasmid DNA (ng / μL) × 10 -9 / (length of plasmid DNA bp×660)]×6.02×10 23 . It was determined that the gene copy numbers of the standard plasmids were 2.9×10 9 copies / μL (pUC57-B646L) and 1.5×10 9 copies / μL (pUC57-MGF505-2R).
Embodiment 3
[0052] The establishment of embodiment 3 double fluorescence quantitative PCR detection method
[0053] (1) The composition of the dual fluorescent quantitative PCR detection kit
[0054] Dual fluorescence quantitative PCR detection kit, including probe method fluorescence quantitative detection reagent (probe method qPCR reaction premix reagent Premix Ex Taq); internal reference dye ROX Reference Dye II; upstream primer B646L-F1, downstream primer B646L-R1, probe Needle B646L-P1, upstream primer MGF505-2R-F1, downstream primer MGF505-2R-R1, probe MGF505-2R-P1; positive control: standard plasmid pUC57-B646L and pUC57-MGF505-2; negative control: double distilled water .
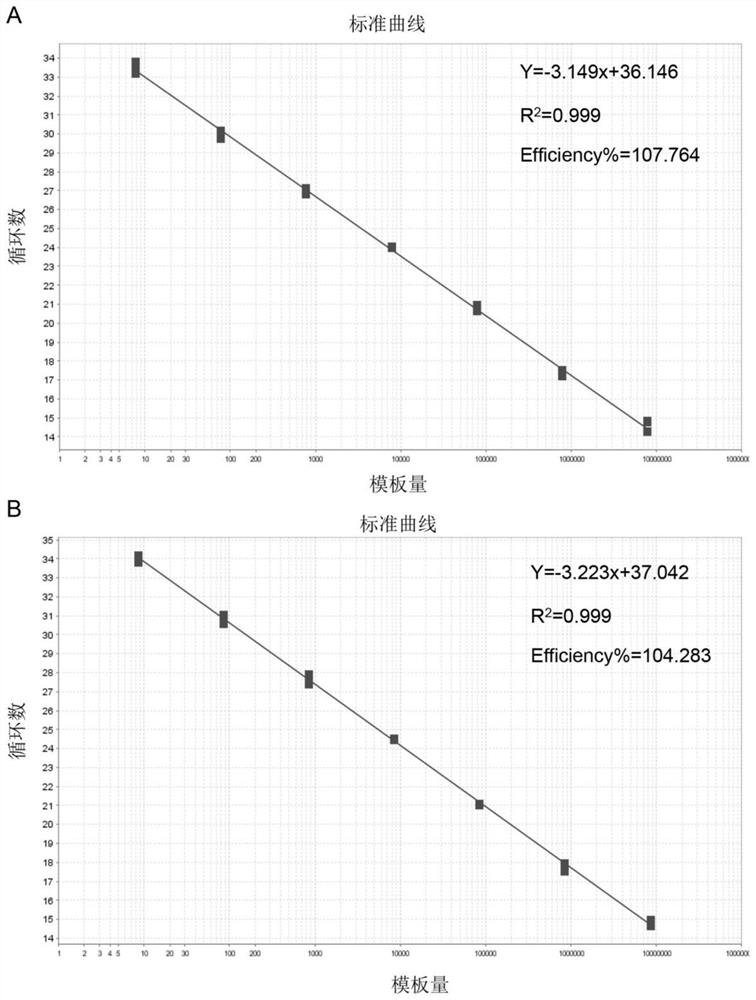
[0055] (2) Establishment of standard curve
[0056] After preliminary screening, it was determined that the reaction system for dual fluorescent quantitative PCR was 25 μL, including 2 μL of the above-mentioned primer mixture and 1 μL of the probe mixture (the final concentration of each primer and probe was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com