Primer, probe and kit for identifying serum I-type Mardivirus gene deletion vaccine, attenuation vaccine and wild virus
A technology of gene deletion vaccine and Marek virus, applied in the direction of microorganism-based methods, microorganisms, biochemical equipment and methods, etc., can solve the problems of indistinguishability, achieve the effect of promoting immune prevention and control, and broad market application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
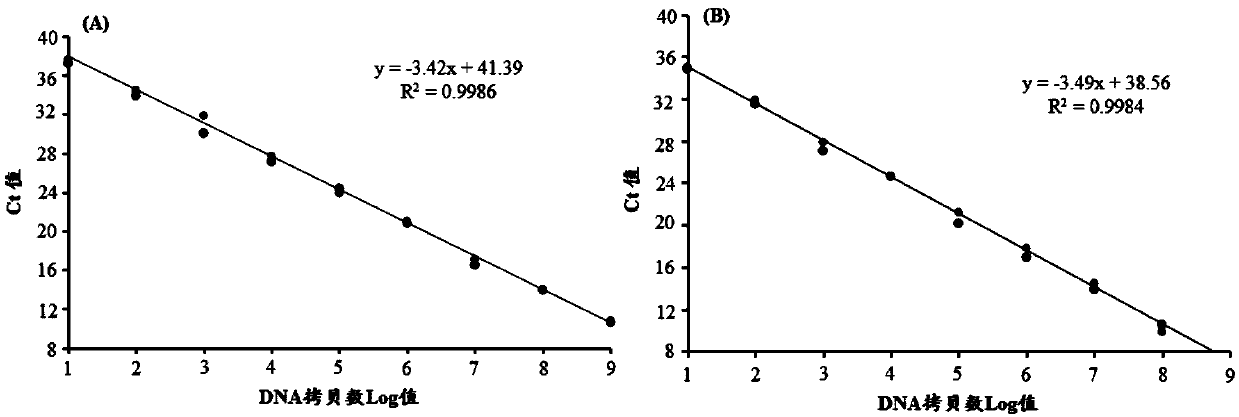
[0070] Embodiment 1: the design of fluorescence quantitative PCR primer and probe
[0071] Through the detailed comparison and analysis of the MDV genome sequence that has been published, especially the cloned and purified MDV genome (the accession numbers of the reference sequences of the present invention in NCBI are respectively: CVI988 / Rispens-DQ530348, GX0101-JX844666, 584A-EU627065, RB1B-EF523390, Md5-AF243438, GA-AF147806, Md11-AY510475, LMS-JQ314003), especially all current MD serum type I gene deletion vaccine genome sequences designed to be able to differentially diagnose serum type I gene deletion vaccine, serum type I Primers and probes of vaccine CVI988 / Rispens and MD wild virus.
[0072] The primer and probe sequences designed to distinguish chicken Marek's disease serum type I gene deletion vaccine from wild virus are as follows:
[0073] Upstream primer: ALMDV-F:5'-CCTACAGTCCCGCTGACGAT-3' (SEQ ID NO.1)
[0074] Downstream primer: ALMDV-R: 5'-CTGTTGGGGATGTCGTG...
Embodiment 2
[0079] Embodiment 2: Establishment of fluorescent quantitative PCR identification method
[0080] 1. Culture of serum type I MDV
[0081] Serum type I vaccine CVI988 / Rispens was purchased from Beijing Lingyu Biotechnology Co., Ltd. Serum type I gene deletion vaccine SC9-1 (recorded in patent CN102628053B), serum type I MDV Md5, GX0101 were all kept by our laboratory. Melt the serum type I MDV frozen in liquid nitrogen quickly in a 37°C water bath, put it into a high-pressure sterilized 1.5ml centrifuge tube in an ultra-clean bench, centrifuge at 2000 rpm for 3 minutes at 4°C, and remove as much as possible. Cell cryopreservation solution, 1ml of cell maintenance solution was gently resuspended and added to the cell bottle in an appropriate amount, placed in a 37°C, 5% carbon dioxide (CO2) incubator for 3-5 days, and after the typical plaques of MDV appeared, follow the Cells were collected by digesting the cells.
[0082] 2. Extraction of MDV DNA
[0083] about 10 7 Add 50...
Embodiment 3
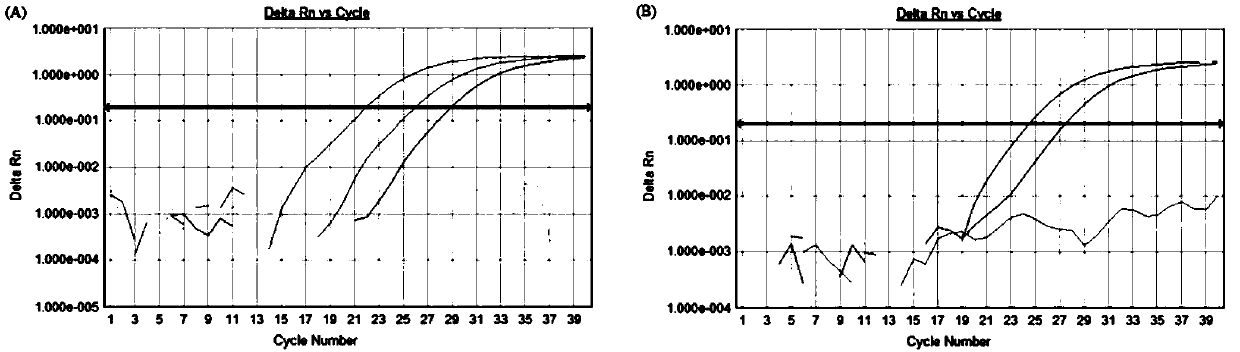
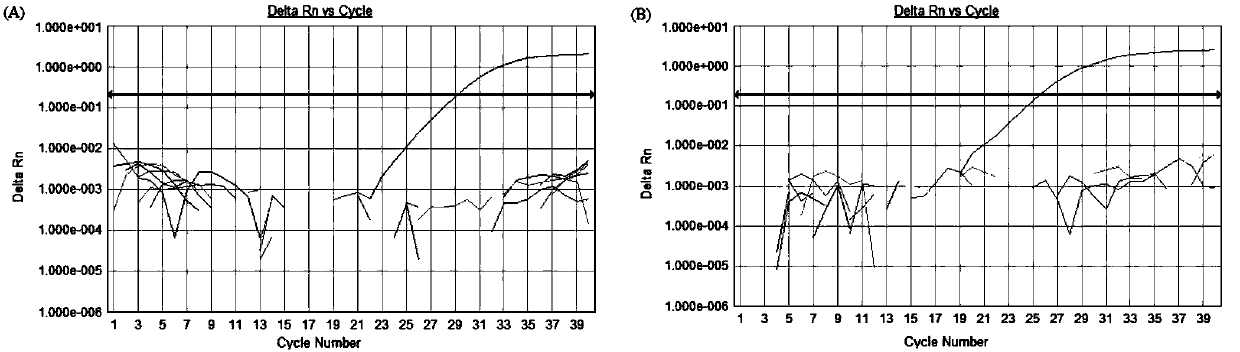
[0097] Example 3: The composition of the kit, the optimization of experimental parameters and the investigation of specificity, sensitivity and repeatability
[0098] 1. The composition of the kit:
[0099] The kit of this embodiment is used for differential diagnosis of serum type I gene deletion vaccine, serum type I vaccine CVI988 / Rispens and MD wild virus, and contains ALMDV detection primers and probes (SEQ ID NO.1-3), FIMDV Detect primers and probes (SEQ ID NO.4-6), positive standard plasmids and fluorescent quantitative PCR reaction reagents.
[0100] Wherein, the positive standard plasmid is pMP recombinant plasmid. The tandem sequence 1324bp of the first 450bp of the MDV meq gene synthesized by a commercial sequencing company and the MDV wild virus pp38 gene was cloned into the pMD19 vector and constructed.
[0101] Fluorescent quantitative PCR reaction reagents include: Gene Expression Master Mix buffer and ddH 2 O.
[0102] 2. Optimization of experimental para...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com