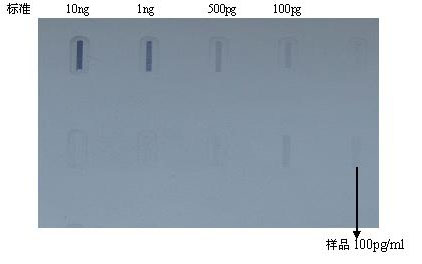
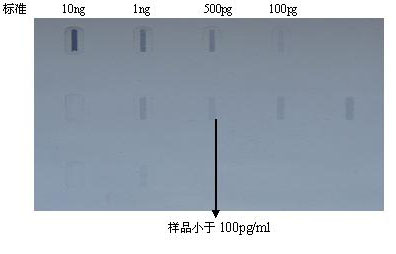
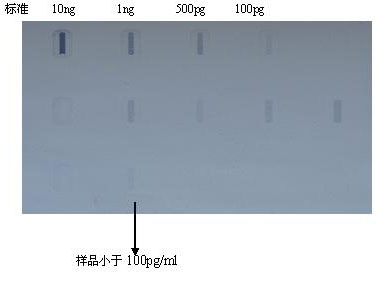
Method for preparing Vero cell influenza virus vaccine
A technology for influenza virus and influenza vaccine, which is used in antiviral agents, pharmaceutical formulations, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Type A virus strain, approved by Who, Solomon strain, A / Solomon / 3 / 06 (H1N1)-, IVR-145, from NIBSC, UK, batch number: Ivr-145, 06 / 234, generation E 7 (Chicken embryo passages are represented by E and numbers, cells are represented by C and numbers) Vero monolayer cells, passage 140-145.
[0038] The A / Solomon / 3 / 06 (H1N1) E7C0 virus seed derived from NIBSC was used to infect Vero cells and chicken embryos for passage, and the passage of the virus seed to E 8 C 0 , the hemagglutination titer was 640, and the passage of Vero cells was E 7 C 1 , The hemagglutination titer is 20-40. E 8 C 0 The Vero cells were infected with the virus species and continued to be subcultured, harvested when the cytopathic changes reached +++-++++, and the main generation seed batch (hemagglutination titer 320) and working seed batch (hemagglutination titer 480) were established and used for Preparation of monovalent vaccines.
Embodiment 2
[0068] A type 3 virus, A / wisconsin / 67 / 2005 (H3N2)-, NYMC-161, from NIBSC, batch number: 06 / 112, generation E 9
[0069] SPF eggs: Beijing Meria Verton Laboratory Animal Technology Co., Ltd.
[0070] Incubate in a biochemical incubator at 37°C for 10 days.
[0071] Vero monolayer cells, passage 140-145;
[0072] A / wisconsin / 67 / 2005 (H3N2)E 9 C 0 Infected chicken embryos derived from NIBSC virus species for passage, the passage of the virus species after passage of chicken embryos is E 10 C 0 , the hemagglutination titer is 480, the virus seed infects Vero cells and continues to be subcultured, and the cell lesion reaches +++-++++ and harvests, and establishes the main generation seed batch (hemagglutination titer 240) and the working seed batch (blood Clotting titer 480), and used in the preparation of monovalent vaccines.
[0073] 225 bottles of monolayer Vero cells 3 liter spinner bottle, cell passage 145, 5 days old, wash the cells three times with washing s...
Embodiment 3
[0101] Type B virus, B / Malaysia / 2506 / 2004, from NIBSC, UK, batch number: 06 / 104, generation E 4
[0102] SPF eggs: Beijing Meria Verton Laboratory Animal Technology Co., Ltd.
[0103] Incubate in a biochemical incubator at 37°C for 10 days.
[0104] Vero monolayer cells, passage 140-145;
[0105] Will B / Malaysia / 2506 / 2004E 4 C 0 Originated from NIBSC virus species infected chicken embryos and Vero cells, the hemagglutination titer reached 240, and the virus generation after passage was E 5 C 0 , while Vero cells were passaged to E 4 C 1 , with a hemagglutination titer of 10. Will B / Malaysia / 2506 / 2004E 4 C 1 Virus seeds are returned to chicken embryos, and the hemagglutination titer can reach 480, and then continue to be passed on Vero cells to establish the main generation seed batch (hemagglutination titer 160) and working seed batch (hemagglutination titer 240), which are used for monovalent vaccines preparation.
[0106] 225 bottles of monolayer Vero ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com